Research group Prof. Dr. Jürgen Janek
Physical chemistry of solids – solid state ionics and electrochemistry
- Current notice
-
Are you interested in joining our group? You can find current vacancies in the job market of JLU Giessen (FB 08, Biologie und Chemie, Physikalisch-Chemisches Institut). You are also welcome to receive further information by e-mail.
- Welcome to our homepage!
- Recent Publications
-
Transition Metal Oxides and Li2CO3 as Precursors for the Synthesis of Ni-Rich Single-Crystalline NCM for Sustainable Lithium-Ion Battery Production
R. Ruess, M. A. Ulherr, E. Trevisanello, S. Schröder, A. Henss, and J. Janek, J. Electrochem. Soc. 169 (2022) 070531; find paper hereDeeper Understanding of the Lithiation Reaction during the Synthesis of LiNiO2 Towards an Increased Production Throughput
P. Kurzhals, F. Riewald, M. Bianchini, S. Ahmed, A. M. Kern, F. Walther, H. Sommer, K. Volz, J. Janek, J. Electrochem. Soc. (2022) 050526; find paper hereA Quasi-Multinary Composite Coating on a Nickel-Rich NCM Cathode Material for All-Solid-State Batteries
D. Kitsche, F. Strauss, Y. Tang, N. Bartnick, A.‐Y. Kim, Y. Ma, C. Kübel, J. Janek, T. Brezesinski, Batter. Supercaps (2022) e202100397; find paper hereIn Situ Investigation of Lithium Metal–Solid Electrolyte Anode Interfaces with ToF‐SIMS
S.‐K. Otto, L. M. Riegger, T. Fuchs, S. Kayser, P. Schweitzer, S. Burkhardt, A. Henss, and J. Janek, Adv. Mater. Interfaces. (2022) 210387; find paper hereInfluence of Lithium Ion Kinetics, Particle Morphology and Voids on the Electrochemical Performance of Composite Cathodes for All-Solid-State Batteries
A. Bielefeld, D. A. Weber, R. Ruess, V. Glavas, and J. Janek, J. Electrochem. Soc. (2022); find paper hereDefect Chemistry of Individual Grains with and without Grain Boundaries of Al-Doped Ceria Determined Using Well-Defined Microelectrodes
J. Zahnow, M. Bastianello, J. Janek, and M. T. Elm, J. Phys. Chem. C (2022); find paper here
- Picture of the month - March 2021
-
Here you can find alternating insights into our research group. Enlarged versions of all published pictures can be found here.
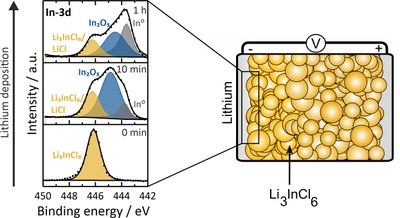
Solid-state batteries have been researched and characterized with greater intensity in recent years due to their better properties compared to lithium-ion batteries, such as higher safety or broader operating temperature and comparable ionic conductivities. To compensate for the higher density of solid electrolytes, using lithium metal as anode material is necessary to obtain good gravimetric and volumetric energy densities. However, lithium metal is very reactive. If electronically conductive products are formed during the reaction of the solid electrolyte with lithium, this electrolyte cannot be in direct contact with lithium, otherwise short circuits may occur.In order to investigate the reaction products of the halide solid electrolyte Li3InCl6 with lithium, lithium is applied to the electrolyte by sputter deposition. In situ X-ray photoelectron spectroscopy (XPS) is used to investigate the resulting decomposition products. It was found that Li3InCl6 decomposes into In2O3 and indium metal, among others. Since indium metal is electronically conductive, the electrolyte will decompose until either Li3InCl6 or the lithium is depleted, thus the electrolyte cannot be used in direct contact with lithium. (Picture submitted by Luise Riegger)
- The WG Janek is involved in the following networks
-