Research
The manifold areas of our research
- Organocatalysis
- Organocatalysis combines the concepts of molecular recognition as well as supramolecular chemistry with enzyme-like catalytic activity. Noting that about half of all enzymes do not carry a metal center it is obvious that this approach has long been underrated. Although this is a new and emerging field, it is already possible to catalyze many types of organic reactions with small, well-designed organic molecules. This circumvents the use of often toxic metals (leading to environmentally benign methods, green chemistry), and the preparation of the catalysts is much easier as it relies on the well-developed synthetic arsenal for tailor-making organic structures. In our group we have developed thiourea-based catalysts that are effective in catalyzing a variety of transformations such as Diels-Alder reactions, acetalization, THP-protection, stereoselective acyl transfer, reductions, epoxide openings and many more. Currently, we develop reactions and reaction sequences utilizing cooperative catalysis and multicatalysis.
- Reactive Intermediates
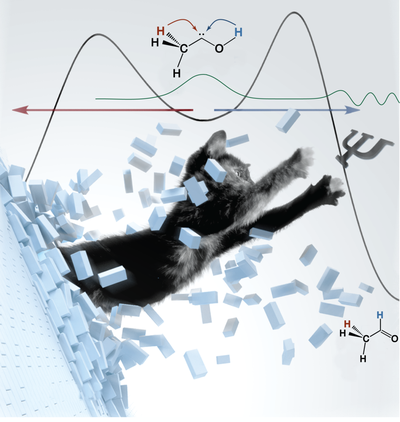
- Singlet carbenes incorporating a divalent carbon atom (R–C–R’) have grown from laboratory curiosities and theoreticians’ pet peeves into reagents in the growing field of stable (i.e., heterocyclic) carbene chemistry. Still, the experimental characterization of many other simple yet fundamentally important reactive species such as alkyl- or hydroxycarbenes, silylenes or sulfenes is hampered by their high reactivity or lack of precursors: Hydroxycarbenes, for instance, have been an unknown class of compounds until 2008, when our group reported the synthesis and characterization of hydroxymethylene (H–C–OH), whose preparation has been challenging organic chemists for more than 80 years. The reaction of hydroxycarbene with formaldehyde would be a source of simple sugars (the so-called “formose reaction” in the origin of life theory). Considerable efforts are ongoing to understand the formation and distribution of a wide variety of simple organics in extraterrestrial environments, and the examination of the structures and reactivities of prototypes such as various hydroxycarbenes, simple carboxylic acids or seemingly "exotic" heteroatom-containing compounds may also provide glimpses of the prebiotic earth.
- Nanodiamonds (Diamondoids)
- Diamondoids (nanometer-sized, hydrogen-terminated diamond hydrocarbons, nanodiamonds) are emerging as complementary materials to fullerenes and carbon nanotubes. In contrast to the latter, nanodiamonds are available in large quantities from crude oil, are chemically well-defined, and of high purity. Nanodiamonds are likely to share some of the unique properties of macroscopic diamond that are very attractive for a number of applications. One of the challenges with these novel building blocks is their selective and preparatively useful C–H bond functionalization. We have developed a variety of such approaches yielding numerous derivatives that can now be utilized for applications including platforms for organocatalysis and, in particular, organic molecular electronics.
- Computational Chemistry
- How does one begin to encapsulate the important aspects of chemistry within one concise frame? A time honored place to start is "from the beginning" or ab initio, as it's said in Latin – the modern chemical terminology refers to high-level computations utilizing state-of-the-art mathematical theory as well as computer equipment. The award of the 1998 Nobel prize for computational chemistry is indicative of the key role that calculation has come to play in contemporary science. High-level ab initio results nowadays approach experimental accuracy. In the pharmaceutical industry, computational chemistry, rational design, and molecular simulation are regarded as mainstream. This important transformation is due, in no small part, to explosive technological advances in the microcomputer hardware and software industries, which have placed powerful computational tools in the hands of everyday scientists. Our research program aims at both using and implementing ab initio as well as density functional theory to challenging chemical problems.
- Tunneling Studies
- While not unknown to the physicist, the fundamental role of quantum mechanical tunneling effects in chemistry has long been neglected at large. Whereas tunneling has traditionally been viewed as an exotic phenomenon that may speed up otherwise slow reactions under conditions that have little to no chemical relevance, the advent of hydroxymethylene (HCOH) has demonstrated that tunneling indeed does not only influence, but may even dominate chemical reactivity entirely. Following the pioneering studies on HCOH, a sizeable number of examples clearly showing the critical importance of the tunneling effect in chemistry has emerged, ranging from other members of the hydroxycarbene family to carboxylic acids and leading to the introduction of the novel concept of Tunneling Control. Building upon our early results, we are aiming to explore the importance of tunneling in biologically important compounds as well as to elucidate the electronic factors that govern it.
- Dispersion
- A Gecko can walk up a smooth glass window because of the adhesion in the millions of hydrophobic setae on its toes that convey van der Waals (vdW) interactions with the surface. The attractive part of such vdW-interactions is an electron correlation (quantum mechanical many-body) effect normally referred to as London dispersion. Its overwhelming role in the formation of condensed matter has been known since the pioneering contributions of J. D. van der Waals and F. London who related dispersion to spectroscopic properties, particularly the polarizability of the interacting partners. In chemistry, dispersion helps rationalize the varying boiling points of alkanes, the greater stability of branched over linear alkanes, the π-π attractive structures of graphite and graphenes, and the mutual attraction of s-π systems. Strong covalent bonding as well as non-covalent interactions such as hydrogen- or halogen-bonding, Pauli repulsion (“steric effects”), and electrostatic multipole interactions are mainly used as “design elements” to affect chemical structures and reactivity. Extending this incomplete view is one of the key goals of the Schreiner Group, with a focus on dispersion interactions as important control elements for structure and reactivity of organic molecules. One of the major objectives is the qualitative determination and quantification of dispersion interactions utilizing a selection of particularly suitable molecular systems. A selection of our work in this arena can be found below.