1,1,2-Ethenetriol: The Enol of Glycolic Acid, a High-Energy Prebiotic Molecule.
Artur Mardyukov, Felix Keul, and Peter R. Schreiner
Angew. Chem. Int. Ed. 2021, 60,15313–15316. DOI: 10.1002/anie.202104436
The simple yet uncharacterized high energy tautomer of glycolic acid, namely 1,1,2-ethenetriol, an important molecule in the abiotic synthesis of sugar acids, has now been identified by IR and UV/Vis spectroscopy. With these data now in hand, it now awaits its identification in space as another building block for prebiotic chemistry.
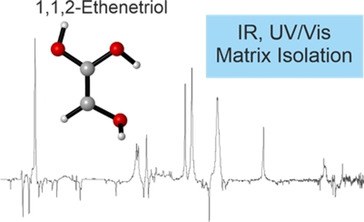
As low-temperature conditions (e.g. in space) prohibit reactions requiring large activation energies, an alternative mechanism for follow-up transformations of highly stable molecules involves the reactions of higher energy isomers that were generated in a different environment. Hence, one working model for the formation of larger organic molecules is their generation from high-lying isomers of otherwise rather stable molecules. As an example, we present here the synthesis as well as IR and UV/Vis spectroscopic identification of the previously elusive 1,1,2-ethenetriol, the higher energy enol tautomer of glycolic acid, a rather stable and hence unreactive biological building block. The title compound was generated in the gas phase by flash vacuum pyrolysis of tartronic acid at 400 °C and was subsequently trapped in argon matrices at 10 K. The spectral assignments are supported by B3LYP/6–311++G(2d,2p) computations. Upon photolysis at λ=180–254 nm, 1,1,2-ethenetriol rearranges to glycolic acid and ketene.
