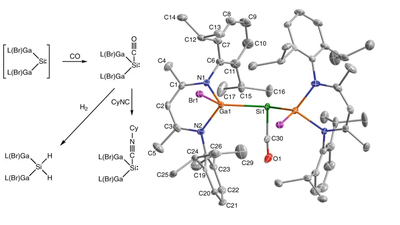
A silicon–carbonyl complex stable at room temperature
Chelladurai Ganesamoorthy, Juliane Schoening, Christoph Wölper, Lijuan Song, Peter R. Schreiner, and Stephan Schulz
Nat. Chem. 2020, 12, 608–614. DOI: 10.1038/s41557-020-0456-x
Highlights: a) Leigh Krietsch Boerner Chem. Eng. News. 2020, 98 (16), 9; b) David Schilter Nat. Rev. Chem. 2020, 4, 274; c) Nachr. Chem. 2020, 68, 42.
Main-group-element compounds with energetically high-lying donor and low-lying acceptor orbitals are able to mimic chemical bonding motifs and reactivity patterns known in transition metal chemistry, including small-molecule activation and catalytic reactions. Monovalent group 13 compounds and divalent group 14 compounds, particularly silylenes, have been shown to be excellent candidates for this purpose. However, one of the most common reactions of transition metal complexes, the direct reaction with carbon monoxide and formation of room-temperature isolable carbonyl complexes, is virtually unknown in main-group-element chemistry. Here, we show the synthesis, single-crystal X-ray structure, and density functional theory computations of a room-temperature-stable silylene carbonyl complex [L(Br)Ga]2Si:–CO (L = HC[C(Me)N(2,6-iPr2-C6H3)]2), which was obtained by direct carbonylation of the electron-rich silylene intermediate [L(Br)Ga]2Si:. Furthermore, [L(Br)Ga]2Si:–CO reacts with H2 and PBr3 with bond activation, whereas the reaction with cyclohexyl isocyanide proceeds with CO substitution.