Spectroscopic Characterization and Photochemistry of the Vinylsulfinyl Radical.
Zhuang Wu, Lina Wang, Bo Lu, André K. Eckhardt, Peter R. Schreiner and Xiaoqing Zeng
PCCP 2021, 23,16307–16315. DOI: 10.1039/d1cp02584h.
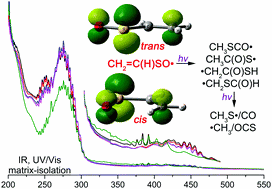
The simplest α,β-unsaturated sulfinyl radical CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)SO˙ has been generated in the gas phase by high-vacuum flash pyrolysis (HVFP) of sulfoxide CH2
C(H)SO˙ has been generated in the gas phase by high-vacuum flash pyrolysis (HVFP) of sulfoxide CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)S(O)CF3 at ca. 800 °C. Two planar cis and trans conformers of CH2
C(H)S(O)CF3 at ca. 800 °C. Two planar cis and trans conformers of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)SO˙ were isolated in cryogenic matrixes (N2, Ne, and Ar) and characterized with IR and UV/Vis spectroscopy. In addition to the photo-induced cis
C(H)SO˙ were isolated in cryogenic matrixes (N2, Ne, and Ar) and characterized with IR and UV/Vis spectroscopy. In addition to the photo-induced cis ![[leftrightharpoons]](https://www.rsc.org/images/entities/char_21cb.gif) trans conformational interconversion, CH2
trans conformational interconversion, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)SO˙ displays complex photochemistry. Upon irradiation with a purple light LED (400 nm), CH2
C(H)SO˙ displays complex photochemistry. Upon irradiation with a purple light LED (400 nm), CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)SO˙ isomerizes to novel radicals CH3SCO˙, ˙CH2SC(O)H, and ˙CH2C(O)SH with concomitant dissociation to a caged molecular complex CH3S˙⋯CO. Subsequent UV-laser (266 nm) irradiation causes fragmentation to ˙CH3/OCS and additional formation of an elusive carbonyl radical CH3C(O)S˙, which rearranges to ˙CH2C(O)SH upon further UV-light irradiation (365 nm). The vibrational data and bonding analysis of the two conformers of CH2
C(H)SO˙ isomerizes to novel radicals CH3SCO˙, ˙CH2SC(O)H, and ˙CH2C(O)SH with concomitant dissociation to a caged molecular complex CH3S˙⋯CO. Subsequent UV-laser (266 nm) irradiation causes fragmentation to ˙CH3/OCS and additional formation of an elusive carbonyl radical CH3C(O)S˙, which rearranges to ˙CH2C(O)SH upon further UV-light irradiation (365 nm). The vibrational data and bonding analysis of the two conformers of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)SO˙ suggest that both are floppy radicals in which the unpaired electron conjugates with the vicinal π(C
C(H)SO˙ suggest that both are floppy radicals in which the unpaired electron conjugates with the vicinal π(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) bond, leading to significant contribution of the canonical resonance form of ˙CH2–C(H)SO. The mechanism for the isomerization of CH2
C) bond, leading to significant contribution of the canonical resonance form of ˙CH2–C(H)SO. The mechanism for the isomerization of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)SO˙ is discussed based on the observed intermediates along with a computed potential energy profile at the CCSD(T)-F12a/aug-cc-pVTZ//B3LYP/6-311++G(3df,3pd) level of theory.
C(H)SO˙ is discussed based on the observed intermediates along with a computed potential energy profile at the CCSD(T)-F12a/aug-cc-pVTZ//B3LYP/6-311++G(3df,3pd) level of theory.