Bis-3-chloropiperidine als DNA-Alkylantien
Synthesis and DNA Cleavage Activity of Bis-3-chloropiperidines as Alkylating Agents
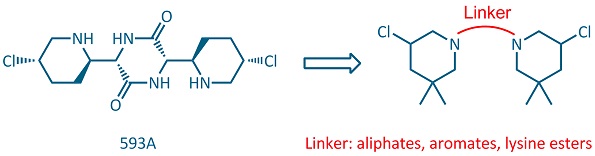
Alkylating agents, like nitrogen mustards, which interact directly with DNA to form covalent bonds have an important therapeutic role in anticancer treatment. The antibiotic 593A, with potential antitumour activity, can be considered as naturally occurring piperidine mustard containing a unique 3-chloropiperidine ring. However, the total synthesis of this antibiotic proved to be rather challenging. With the aim of designing simplified analogues of this natural product, we developed an efficient bidirectional synthetic route to bis-3-chloropiperidines joined by flexible, conformationally restricted, rigid diamine or lysine linkers.[1,2] On a second attempt we also examined the influence of different substituents on the side chain of those lysine[2,3] and benzoic acid linkers.
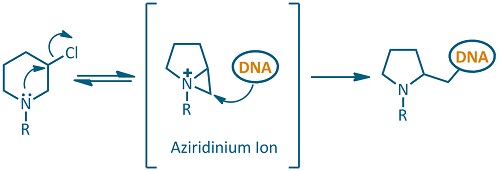
The mechanism of action is based on the formation of a highly reactive electrophilic aziridinium ion. This reactive species can be readily attacked by nucleophilic centers of the DNA bases. In the first place, base alkylation prevents DNA replication and induces DNA fragmentation by hydrolytic reactions, which ultimately leads to cell death.
Our studies on the synthesis and biological evaluation of bis-3-chloropiperidines as DNA alkylating agents demonstrate that these mustard-like compounds alkylate DNA very effectively.[1-3]
[1] I. Zuravka, R. Roesmann, A. Sosic, W. Wende, A. Pingoud, B. Gatto, R. Göttlich, ChemMedChem 2014, 9, 2178–2185.
[2] I. Zuravka, R. Roesmann, A. Sosic, R. Göttlich, B. Gatto, Bioorg.Med.Chem. 2015,23, 1241–1250.
[3] I. Zuravka, A. Sosic, B. Gatto, R. Göttlich, Bioorganic & medicinal chemistry letters 2015, 25, 4606.
| Project-Director: | Prof. Dr. Richard Göttlich |
|---|---|
| Co-Worker: | Dr. Ivonne Zuravka |
| M.Sc. Alexander Francke | |
| M.Sc. Tim Helbing |
