Methylenverbrückte N-Heterocyclen als neue chirale Liganden
New C-bridged N-heterocycles as chiral ligands
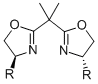
The concept of C2-symmetry has proved to be invaluable for asymmetric catalysis. Among the vast number of ligands known, the most successful ones such as the binaphthyls or the bisoxazolines contain a C2-symmetry axis, and only a few notable exceptions such as phosphinooxazolines or ferrocenyl ligands are equally effective chiral inductors.
|
1 |
~ |
2 |
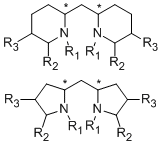
As the fact that C2-symmetrical bisoxazolines 1 are excellent ligands, we were interested in similar methylen-bridged N-heterocycles 2 as new chiral ligands. In comparison to the bisoxazoline nitrogen the bispiperidine- and bispyrollidine-nitrogen is a better donor, due to its hybridization, thus different complexation behavior of these ligands can be expected. Furthermore, by varying the substituents attached to the nitrogen, the electronics and sterics of the ligand can be triggered.
Copper dioxygen complexes with some of these ligands
Copper is known as active center of several redox active metalloproteins. Because of the ability of many copper enzymes to catalyze selective oxidations with air, one of the goals in bioinorganic chemistry is to model this functionality and to apply such small molecular synthetic copper complexes as catalysts for selective oxidation reactions.
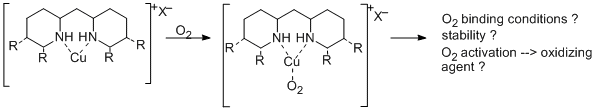
To study a couple of such model complexes, they are synthesized and characterized using our new N-heterocyclic ligands.
| Projekt director: | Prof. Dr. Richard Göttlich |
| Co-worker: | M.Sc. Tim-Daniel Stumpf |