Synthese von großen unsymmetrischen Iminen mittels Palladium katalysierter Kreuzkupplungsreaktion
The synthesis of large unsymmetrical imines
by palladium-catalyzed cross-coupling
The use of palladium-catalyzed cross-coupling reactions to build aromatic compounds is a long standing method in organic synthesis with many literature references.[1] Although many variations exist, the basic model for the reactions can be simplified to:
|
Scheme 1: Basic Model for the palladium-catalyzed cross-coupling |
The possibility of a selective forumylation by means of cross coupling has aroused much interest. Especially the research groups around Heck, Sonogashira, Suzuki, and Hiyama have studied in detail the carbonylative reactions involving palladium[3]. We are looking for an efficient way to prepare imines instead of ketones via a Pd-catalysed coupling reaction.
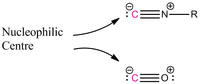
A first step to synthesizing bulky unsymmetrical immines was to find an analoug of the carbonyl group which could be used for the formylation reaction. The isonitrile functionality is isoelectronic to the carbonyl group, and we supposed that these molecules would react in a similar fashion.
A siginificant advantage of using isonitriles is that they are generally liquids, and are much easier to handle than CO.[4] Furthermore the substituents of the isonitrile can be used to trigger the speed of the insertion reaction. In this fashion electron rich and poor, stabilized or bulky isonitriles can be used. A small selection of possible isonitriles is:
|
 |
|
|
Scheme 2: Isonitrile vs. CO |
Scheme 3: Possible Isonitriles |
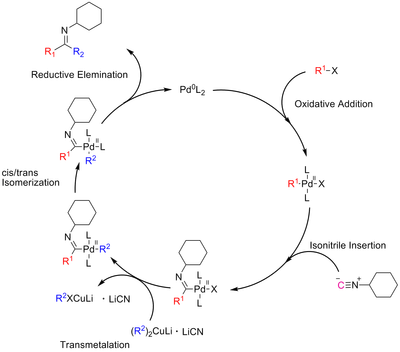
A proposed mechanism for the cross-coupling with isonitrile insertion is show in scheme 4[2]:
|
Scheme 4: A possible mechanism for the cross-coupling with the isonitrile insertion |
First promising results applying this concept have been accomplished using higher order cuprates and cyclohexylisocynanid, with isolated yields of imines of up to 48%. Future work focus on optimizing this reaction.
[1] Bifu Liu, Yibiao Li, Huanfeng Jiang,Meizhou Yin, Huawen. 2012. Advanced Synthesis & Catalysis, 11-12, 2288–2300.
[2] Johannes G.P. Delis, Peter G.Aubel, Piet W.N. m. van Leeuwen, Kees Vrieze, Nora Veldman, Anthony L. Spek. Journal of the Chemical Society, Chemical Communications 1995, 21, 2163–2247.
[3] Nuria Rodriguez and Lukas J. Goossen. Chem. Soc. Rev., 2011, 40, 5030–5048.
[4] Xiao-Feng Wu, Helfried Neumann and Matthias Beller. Chem. Soc. Rev., 2011, 40, 4986–5009.