Electronic Stabilization of Ground State Triplet Carbenes.
Adelina Nemirowski and Peter R. Schreiner, J. Org. Chem. 2007, 72, 9533. Download
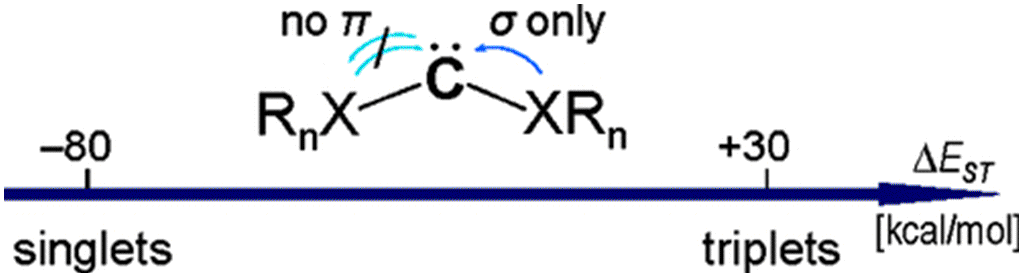
Abstract. Based on systematic ab initio (CCSD(T)/cc-pVDZ) studies of substituent effects, we present a concept for the construction of electronically stabilized triplet ground state carbenes with singlet−triplet energy separations (∆EST) exceeding that of methylene. Sterically demanding and conjugating substituents were excluded from the selection of model compounds under investigation, as these either destabilize both the singlet and the triplet states or delocalize unpaired spins away from the carbene carbon. Negative partial charges on the carbene center allow for the prediction of the electronic stabilization of substituted carbenes. To decrease carbene reactivity, we chose b-substituents with strong polar bonds. Among them, highly electronegative elements such as fluorine and oxygen enlarge the ∆EST value with respect to hydrogen, while chlorine does not due to p-orbital participation.