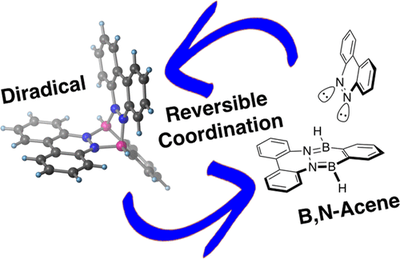
A Stable Organic Neutral Diboron Diradical via Reversible Coordination
Zhenpin Lu, Henrik Quanz, Olaf Burghaus, Jonas Hoffmann, Christian Logemann, Peter R. Schreiner, Sebastian Beeck and Hermann A. Wegner
J. Am. Chem. Soc. 2018, 139, 18488–18491. DOI: 10.1021/jacs.7b11823
We report the formation of a stable neutral diboron diradical simply by coordination of an aromatic dinitrogen compound to an ortho-phenyldiborane. This process is reversible upon addition of pyridine. The diradical species is stable above 200 °C. Computations are consistent with an open-shell triplet diradical with a very small open-shell singlet–triplet energy gap that is indicative of the electronic disjointness of the two radical sites. This opens a new way of generating stable radicals with fascinating electronic properties useful for a large variety of applications.