Forming Stereogenic Centers in Acyclic Systems from Alkynes
Roxane Vabre, Biana Island, Claudia Diehl, Peter R. Schreiner, and Ilan Marek*
Angew. Chem. Int. Ed. 2015, 54, 9996–9999. DOI: 10.1002/anie.201504756
Noted as very important paper (top 5% of all Angewandte publications)
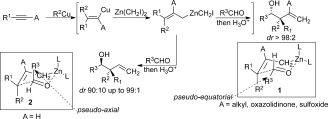
The combined carbometalation/zinc homologation followed by reactions with α-heterosubstituted aldehydes and imines proceed through a chair-like transition structure with the substituent of the incoming aldehyde residue preferentially occupying a pseudo-axial position to avoid the two gauche interactions. The heteroatom in the axial position produces a chelated intermediate (and not a Cornforth–Evans transition structure for α-chloro aldehydes and imines) leading to a face differentiation in the allylation reaction. This method provides access to functionalized products in which three new carbon–carbon bonds and two to three stereogenic centers, including a quaternary one, were created in acyclic systems in a single-pot operation from simple alkynes.
