Combined ab initio molecular dynamics and experimental studies show that carbon atom addition to benzene.
Michael L. McKee, Hans-Peter Reisenauer, and Peter R. Schreiner,
J. Phys. Chem. A. 2014, 118, 2801–2809. DOI: 10.1021/jp501107b.
Car–Parrinello molecular dynamics was used to explore the reactions between triplet and singlet carbon atoms with benzene. The computations reveal that, in the singlet C atom reaction, products are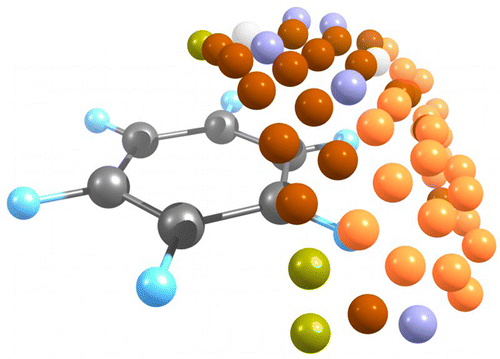 very exothermic where nearly every collision yields a product that is determined by the initial encounter geometry. The singlet C atom reaction does not follow the minimum energy path because the bimolecular reaction is controlled by dynamics (i.e., initial orientation of encounter). On the other hand, in a 10 K solid Ar matrix, ground state C(3P) atoms do tend to follow RRKM kinetics. Thus, ab initio molecular dynamics (AIMD) results indicate that a significant fraction of C–H insertion occurs to form phenylcarbene whereas, in marked contrast to previous theoretical and experimental conclusions, the Ar matrix isolation studies indicate a large fraction of direct cycloheptatetraene formation, without the intermediacy of phenylcarbene. The AIMD calculations are more consistent with vaporized carbon atom experiments where labeling studies indicate the initial formation of phenylcarbene. This underlines that the availability of thermodynamic sinks can completely alter the observed reaction dynamics.
very exothermic where nearly every collision yields a product that is determined by the initial encounter geometry. The singlet C atom reaction does not follow the minimum energy path because the bimolecular reaction is controlled by dynamics (i.e., initial orientation of encounter). On the other hand, in a 10 K solid Ar matrix, ground state C(3P) atoms do tend to follow RRKM kinetics. Thus, ab initio molecular dynamics (AIMD) results indicate that a significant fraction of C–H insertion occurs to form phenylcarbene whereas, in marked contrast to previous theoretical and experimental conclusions, the Ar matrix isolation studies indicate a large fraction of direct cycloheptatetraene formation, without the intermediacy of phenylcarbene. The AIMD calculations are more consistent with vaporized carbon atom experiments where labeling studies indicate the initial formation of phenylcarbene. This underlines that the availability of thermodynamic sinks can completely alter the observed reaction dynamics.
