Phenylsulfinyl Radical: Gas-Phase Generation, Photoisomerization and Oxidation
Jian Xu, Zhuang Wu, Huabin Wan, Guohai Deng, Bo Lu, André K. Eckhardt, Peter R. Schreiner, Tarek Trabelsi, Joseph S. Francisco and Xiaoqing Zeng
J. Am. Chem. Soc. 2018, 140, 9972–9978. DOI: 10.1021/jacs.8b05055
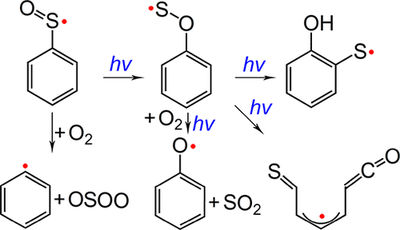
Arylsulfinyl radicals are key intermediates in sulfoxide chemistry. The parent molecule, phenylsulfinyl radical PhSO•, has been generated for the first time in the gas phase through high-vacuum flash pyrolysis of PhS(O)R (R = CF3 and Cl) at about 1000 K. Upon UV light irradiation (365 nm), PhSO• isomerizes to novel oxathiyl radical PhOS• in cryogenic matrices (2.8 K). Prolonged irradiation causes further isomerization of PhOS• to 2-hydroxyphenylthiyl radical, the formation of which has been also observed in the 193 nm laser photolysis of matrix-isolated 2-hydroxybenzenethiol. Concomitantly, ring-opening occurs during the UV photolysis of PhOS• and 2-hydroxybenzenethiol and forms an acyclic thioketoketene radical. Phenylsulfinyl radical reacts partially with molecular oxygen in the gas phase and yields phenyl radical Ph• and OSOO. Upon irradiation (365 nm), the isomeric oxathiyl radical also combines O2 with immediate dissociation to phenoxy radical PhO• and SO2. The identification of the intermediates with IR and UV–vis spectroscopy is supported by quantum chemical computations at the B3LYP/def2-TZVPP and UCCSD(T)/aug-cc-pV(D+d)Z levels of theory. The isomerization of PhSO• has been discussed based on the computed potential energy profile and the comparison with the intensively explored photochemistry of phenylperoxy radical PhOO•.
