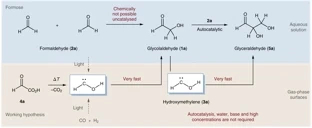
Gas-phase sugar formation using hydroxymethylene as the reactive formaldehyde isomer.
André K. Eckhardt, Michael M. Linden, Raffael C. Wende, Bastian Bernhardt and Peter R. Schreiner
Nature Chem. 2018, 10, 1141–1147. DOI: 10.1038/s41557-018-0128-2
Carbohydrates (CH2O)n are the formal adducts of carbon (atoms) to water with a repeating unit that structurally resembles H–C̈–OH (hydroxymethylene). Although hydroxymethylene has been suggested as a building block for sugar formation, it is a reactive species that had escaped detection until recently. Here we demonstrate that formaldehyde reacts with its isomer hydroxymethylene to give glycolaldehyde in a nearly barrierless reaction. This carbonyl–ene-type transformation operates in the absence of base and solvent at cryogenic temperatures similar to those found in extraterrestrial environments or interstellar clouds. Hydroxymethylene acts as a building block for an iterative sugar synthesis, as we demonstrate through the formation of the triose glyceraldehyde. The thermodynamically preferred ketose dihydroxyacetone does not form, and the formation of further branched sugars in the iterative synthesis presented here is unlikely. The results therefore provide a link between the well-known formose (Butlerow) reaction and sugar formation under non-aqueous conditions.