Computational Chemistry
How does one begin to encapsulate the important aspects of chemistry within one concise frame? A time honored place to start is "from the beginning" or ab initio, as it's said in Latin – the modern chemical terminology refers to high-level computations utilizing state-of-the-art mathematical theory as well as computer equipment. The award of the 1998 Nobel prize for computational chemistry is indicative of the key role that calculation has come to play in contemporary science. High-level ab initio results nowadays approach experimental accuracy. In the pharmaceutical industry, computational chemistry, rational design, and molecular simulation are regarded as mainstream. This important transformation is due, in no small part, to explosive technological advances in the microcomputer hardware and software industries, which have placed powerful computational tools in the hands of everyday scientists. Our research program aims at both using and implementing ab initio as well as density functional theory to challenging chemical problems.
- Director: Univ.-Prof. Peter R. Schreiner
-
Co-workers:
- Computational Chemistry
-
Current work:
Determination of the Absolute Configurations of Chiral Alkanes – An Analysis of the Available Tools. Fumito Saito and Peter R. Schreiner
Eur. J. Org. Chem. 2020, 40, 6328-6339. DOI: 10.1002/ejoc.202000711This article also appears in: ADUC Prize Winners, Noncovalent InteractionsHandy it is to know how to determine the absolute configurations of even the most challenging chiral molecules, in particular alkanes, which are reviewed here. An evaluation of common analytical methods for absolute configuration determination provides a guide for experimental chemists to analyze their own molecules of interest.
Knowledge of absolute configurations (ACs) is crucial to understand the properties and functions of chiral molecules. Although the development of asymmetric reactions has enabled access to a large variety of enantioenriched compounds, stereochemical assignments of chiral molecules, especially chiral alkanes, remain challenging. In this Minireview, we first summarize common analytical techniques for AC determination with a focus on chiral alkanes as analytical targets. Next, we describe some of our efforts over the last 20 years on syntheses and AC determination of chiral alkanes including [123]tetramantane, hexadeuterated neopentane, and trans‐perhydroazulene. Lastly, microscopic techniques and the crystalline sponge method are discussed as particularly promising approaches to revolutionize the field of stereochemical analysis.
The Absolute Configuration of trans-Perhydroazulene. Fumito Saito, Dennis Gerbig, Jonathan Becker and Peter R. Schreiner
Org. Lett. 2020, 22, 3895–3899. DOI: 10.1021/acs.orglett.0c01184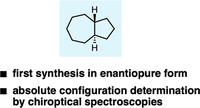
Synthesis and Conformational Analysis of Parent Perhydroazulenes Reveals an Energetically Preferred cis Ring Fusion. Fumito Saito, Jonathan Becker and Peter R. Schreiner
J. Org. Chem. 2020, 85, 4441–4447. DOI: 10.1021/acs.joc.0c00167
TUNNEX: An easy-to-use Wentzel-Kramer-Brillouin (WKB) implementation to compute tunneling half-lives. Hendrik Quanz and Peter R. Schreiner
J. Comput. Chem. 2019, 41, 543-547. DOI: 10.1002/jcc.25711Tunneling in experiments (TUNNEX) is a free open‐source program with an easy‐to‐use graphical user interface to simplify the process of Wentzel‐Kramers‐Brillouin (WKB) computations. TUNNEX aims at experimental chemists with basic knowledge of computational chemistry, and it offers the computation of tunneling half‐lives, visualization of data, and exporting of graphs. It also provides a helper tool for executing the zero‐point vibrational energy correction along the path. The program also enables computing high‐level single points along the intrinsic reaction path. TUNNEX is available at https://github.com/prs-group/TUNNEX. As the WKB approximation usually overestimates tunneling half‐lives, it can be used to screen tunneling processes before proceeding with elaborate kinetic experiments or higher‐level tunneling computations such as instanton theory and small curvature tunneling approaches. © 2018 Wiley Periodicals, Inc.
Computational Chemistry: The fate of current methods and future challenges. Stefan Grimme and Peter R. Schreiner
Angew. Chem. Int. Ed. 2018, 57, 4170–4176. Angew. Chem. 2018, 130, 4241–4248. DOI: 10.1002/anie.201709943
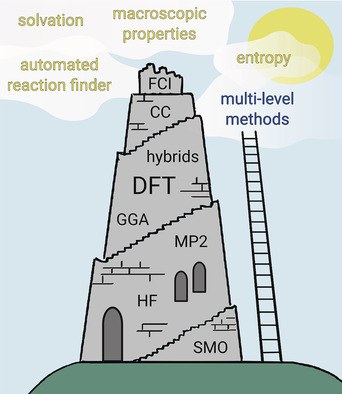
“Where do we go from here?” is the underlying question regarding the future (perhaps foreseeable) developments in computational chemistry. Although this young discipline has already permeated practically all of chemistry, it is likely to become even more powerful with the rapid development of computational hard‐ and software.
Polytriangulane. Wesley D. Allen, Henrik Quanz, and Peter R. Schreiner
J. Chem. Theory Comput. 2016, 12, 4707–4716. DOI: 10.1021/acs.jctc.6b00669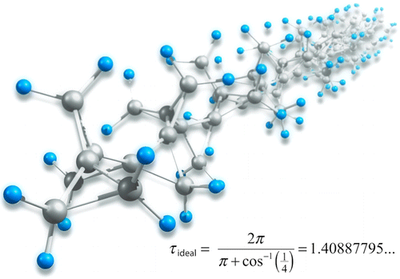
 . A focal point analysis of the ring opening of cyclopropane to propene employing basis sets as large as cc-pCV5Z and correlation treatments as extensive as CCSDT(Q) yields ΔfH0°(cyclopropane) = 17.2 ± 0.1 kcal mol–1. Subsequent application of CCSD(T)/CBS theory to a homodesmotic equation for ring aggregation predicts that ΔfH0° = +16.1 kcal (mol CH)−1 for polytriangulane; hence, this compound is much more stable thermodynamically than acetylene. Similar computations on another hypothetical homodesmotic transformation indicate that the total strain energy in polytriangulane is 42.7 kcal per mole of cyclopropane units.
. A focal point analysis of the ring opening of cyclopropane to propene employing basis sets as large as cc-pCV5Z and correlation treatments as extensive as CCSDT(Q) yields ΔfH0°(cyclopropane) = 17.2 ± 0.1 kcal mol–1. Subsequent application of CCSD(T)/CBS theory to a homodesmotic equation for ring aggregation predicts that ΔfH0° = +16.1 kcal (mol CH)−1 for polytriangulane; hence, this compound is much more stable thermodynamically than acetylene. Similar computations on another hypothetical homodesmotic transformation indicate that the total strain energy in polytriangulane is 42.7 kcal per mole of cyclopropane units.Heats of formation of platonic hydrocarbon cages by means of high-level thermochemical procedures.
Amir Karton, Peter R. Schreiner and Jan M. L. Martin
J. Comput. Chem. 2016, 37, 49–58. DOI: 10.1002/jcc.23963Hydrocarbon cages are key reference materials for the validation and parameterization of computationally cost-effective procedures such as density functional theory (DFT), semiempirical molecular orbital theory, and molecular mechanics. We obtain accurate total atomization energies (TAEs) and heats of formation (ΔfH°298) for platonic and prismatic hydrocarbon cages by means of the Wn-F12 explicitly correlated thermochemical protocols. We consider the following kinetically stable (CH)n polycyclic hydrocarbon cages: (i) platonic hydrocarbons (tetrahedrane, cubane, and dodecahedrane), (ii) prismatic hydrocarbons (triprismane, cubane, and pentaprismane), and (iii) one truncated tetrahedrane (octahedrane). Our best theoretical heat of formation for cubane (144.8 kcal mol−1) suggests that the experimental value adopted by the NIST thermochemical database (142.7 ± 1.2 kcal mol−1) should be revised upwards by ∼2 kcal mol−1. Our best heat of formation for dodecahedrane (20.2 kcal mol−1) suggests that the semiexperimental value (22.4 ± 1 kcal mol−1) should be revised downward by ∼2 kcal mol−1. We use our benchmark Wn-F12 TAEs to evaluate the performance of a variety of computationally less demanding composite thermochemical procedures. These include the Gaussian-n (Gn) and the complete basis set (CBS) methods. The CBS-QB3 and CBS-APNO procedures show relatively poor performance with root-mean-squared deviations (RMSDs) of 4.2 and 2.5 kcal mol−1, respectively. The best performers of the Gn procedures are G4 and G3(MP2)B3 (RMSD = 0.5 and 0.6 kcal mol−1, respectively), while the worst performers are G3 and G4(MP2)-6X (RMSD = 2.1 and 2.9 kcal mol−1, respectively). Isodesmic and even homodesmotic reactions involving these species are surprisingly challenging targets for DFT computations.
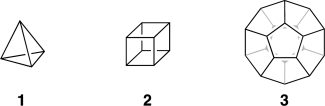
Platonic hydrocarbons: Tetrahedrane (1, C4H4), cubane (2, C8H8), and dodecahedrane (3, C20H20)
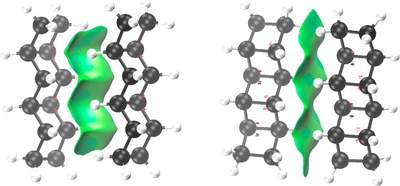
Nature Utilizes Unusual High London Dispersion Interactions for Compact Membranes Comprised of Molecular Ladders. J. Philipp Wagner and Peter R. Schreiner* J. Chem. Theory Comput. 2014, 10, 1353–1358. DOI: 10.1021/ct5000499
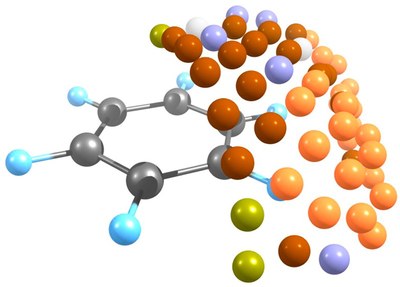
Combined ab initio molecular dynamics and experimental studies show that carbon atom addition to benzene. Michael L. McKee,* Hans-Peter Reisenauer, and Peter R. Schreiner,* J. Phys. Chem. A. 2014, 118, 2801–2809. DOI: dx.doi.org/10.1021/jp501107b
Polytwistane. Shiblee Barua, Henrik Quanz, Martin Olbrich, Peter R. Schreiner, Dirk Trauner, and Wesley D. Allen* Chem. Eur. J. 2014, 20, 1638–1645. DOI: 10.1002/chem.201303081.
Twistane, C10H16, is a classic D2-symmetric chiral hydrocarbon that has been studied for decades due to its fascinating stereochemical and thermodynamic properties. Here we propose and analyze in detail the contiguous linear extension of twistane with ethano (ethane-1,2-diyl) bridges to create a new chiral, C2-symmetric hydrocarbon nanotube called polytwistane. Polytwistane, (CH)n, has the same molecular formula as polyacetylene but is composed purely of C(sp3)
![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H units, all of which are chemically equivalent. The polytwistane nanotube has the smallest inner diameter (2.6 Å) of hydrocarbons considered to date. A rigorous topological analysis of idealized polytwistane and a C236H242 prototype optimized by B3LYP density functional theory reveals that the polymer has a nonrepeating, alternating σ-helix, with an irrational periodicity parameter and an instantaneous rise (or lead) angle near 15 °. A theoretical analysis utilizing homodesmotic equations and explicit computations as high as CCSD(T)/cc-pVQZ yields the enthalpies of formation
H units, all of which are chemically equivalent. The polytwistane nanotube has the smallest inner diameter (2.6 Å) of hydrocarbons considered to date. A rigorous topological analysis of idealized polytwistane and a C236H242 prototype optimized by B3LYP density functional theory reveals that the polymer has a nonrepeating, alternating σ-helix, with an irrational periodicity parameter and an instantaneous rise (or lead) angle near 15 °. A theoretical analysis utilizing homodesmotic equations and explicit computations as high as CCSD(T)/cc-pVQZ yields the enthalpies of formation  (twistane)=−1.7 kcal mol−1 and
(twistane)=−1.7 kcal mol−1 and  (polytwistane)= +1.28 kcal (mol CH)−1, demonstrating that the hypothetical formation of polytwistane from acetylene is highly exothermic. Hence, polytwistane is synthetically viable both on thermodynamic grounds and also because no obvious pathways exist for its rearrangement to lower-lying isomers. The present analysis should facilitate the preparation and characterization of this new chiral hydrocarbon nanotube.
(polytwistane)= +1.28 kcal (mol CH)−1, demonstrating that the hypothetical formation of polytwistane from acetylene is highly exothermic. Hence, polytwistane is synthetically viable both on thermodynamic grounds and also because no obvious pathways exist for its rearrangement to lower-lying isomers. The present analysis should facilitate the preparation and characterization of this new chiral hydrocarbon nanotube.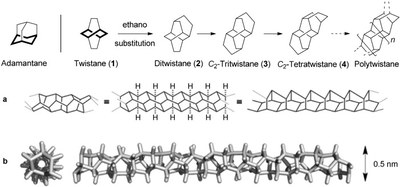
Steric crowding can stabilize a labile molecule: Solving the hexaphenylethane riddle. Stefan Grimme* and Peter R. Schreiner*
Angew. Chem. Int. Ed. 2011, 50, 12639–12642. Angew. Chem. 2011, 123, 12849–12853. Download Highlight: Nachr. Chem. 2012, 60, 108.
Abstract. 12 not so angry men: Hexaphenylethane is unstable, a phenomenon traditionally attributed to steric repulsion between the six phenyl rings. However, adding 12 bulky tert-butyl groups, one to each of the 12 meta positions, gives a stabile ethane derivative (see space-filling model and potential energy curve for the dissociation of the central C
![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) C bond). This unexpected stabilization is shown to result from attractive dispersion interactions between the substituents.
C bond). This unexpected stabilization is shown to result from attractive dispersion interactions between the substituents.σ/σ- and π/π-Interactions are Equally important: Multilayered Graphanes. Andrey A. Fokin,* Dennis Gerbig, and Peter R. Schreiner*
J. Am. Chem. Soc. 2011, 133, 20036–20039. Download
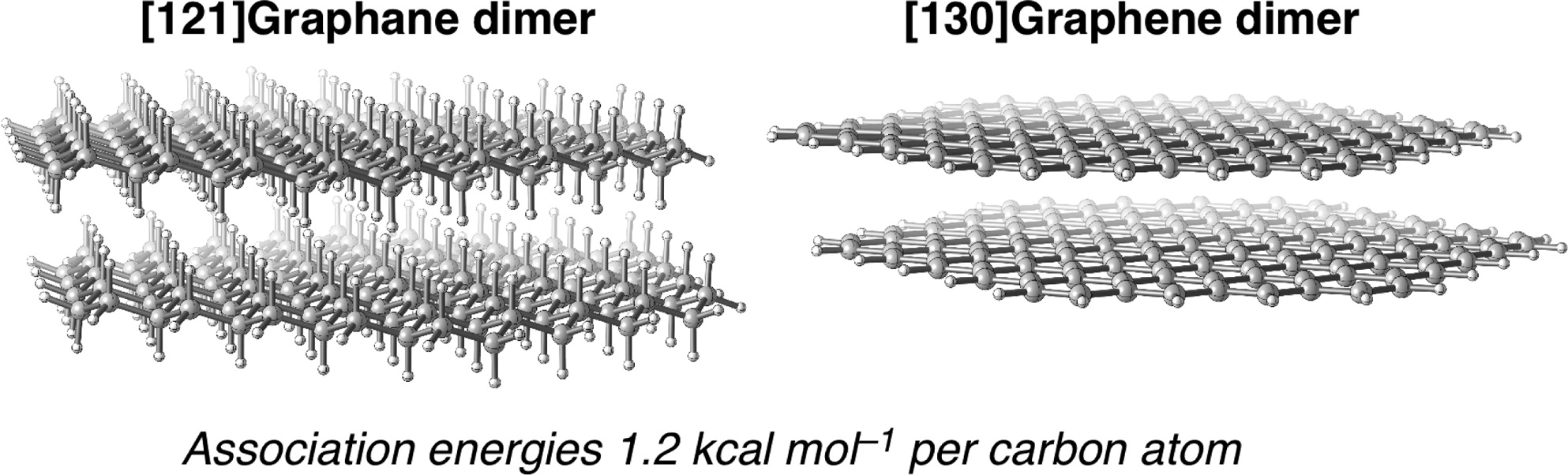
Abstract. The properties of single-sheet [n]graphanes, their double-layered forms (diamondoids), and their van der Waals (vdW) complexes (multilayered [n]graphanes) were studied for n = 10–97 at the dispersion-corrected density functional theory (DFT) level utilizing B97D with a 6-31G(d,p) basis set; for comparison, we also computed a series of structures at M06-2X/6-31G(d,p) as well as B3LYP-D3/6-31G(d,p) and evaluated SCS-MP2/cc-pVDZ single-point energies. The association energies for the vdW complexes reach 120 kcal mol–1 already at 2 nm particle size ([97]graphane dimer), and graphanes adopt layered structures similar to that of graphenes. The association energies of multilayered graphanes per carbon atom are rather similar and independent of the number of layers (ca. 1.2 kcal mol–1). Graphanes show quantum confinement effects as the HOMO–LUMO gaps decrease from 8.2 eV for [10]graphane to 5.7 eV for [97]graphane, asymptotically approaching 5.4 eV previously obtained for bulk graphane. Similar trends were found for layered graphanes, where the differences in the electronic properties of double-sheet CH/σ vdW and double-layered CC/σ diamondoids vanish at particles sizes of 1 nm. For comparison, we studied the parent CC/π systems, i.e., the single- and double-sheet [n]graphenes (n = 10–130) for which the association energies demonstrate the same trends as in the case of [n]graphanes; in both cases the band gaps decrease with an increase in system size. The [112]graphene dimer (HOMO–LUMO gap = 0.5 eV) already approaches the 2D metallic properties of graphite.
Understanding the Torquoselectivity in 8π-Electrocyclic Cascade Reactions: Synthesis of Fenestradienes versus Cyclooctatrienes. Catherine Hulot, Shadi Amiri,Gaëlle Blond, Peter R. Schreiner, and Jean Suffert.
J. Am. Chem. Soc. 2009, 131, 13387. Download
Abstract. Unusual and novel polycyclic cyclooctatrienes, fenestradienes, and fenestrenes form readily from trienynes depending on the structure of the starting trienynes and the reaction conditions. The experimentally observed high torquoselectivities and complete diastereoselectivities of the 8π-electrocyclization products have been thoroughly studied using density functional computations at B3PW91/6-31g(d,p). The different P- and M-helical topologies for the Möbius aromatic transition structures are the origin of the observed torquoselectivities in the cyclooctatrienes. The P-helical topologies direct the newly formed single bonds into a favorable equatorial position of the neighboring cycloalkane moieties (X = ring size) that retain their most stable conformation. The M-helical transition structures lead to an axial connection for the smaller rings (X = 4–6) and an equatorial connection for the seven- and eight-membered cycloalkanes. This leads to unfavorable conformations for the larger cycloalkane moieties. Experiments and computations show that for trienynes involving small neighboring cycloalkane groups (X = 4–6) M-helical topology is preferred toward cyclooctatrienes and in the following the corresponding fenestradienes can be formed as kinetic or even thermodynamic products; they convert to their more stable cyclooctatriene valence isomers derived from P-helical transition structures at higher temperatures. For larger cycloalkane moieties with more conformational flexibility only cyclooctatrienes with torquoselectivities derived from P-helical transition structures form.

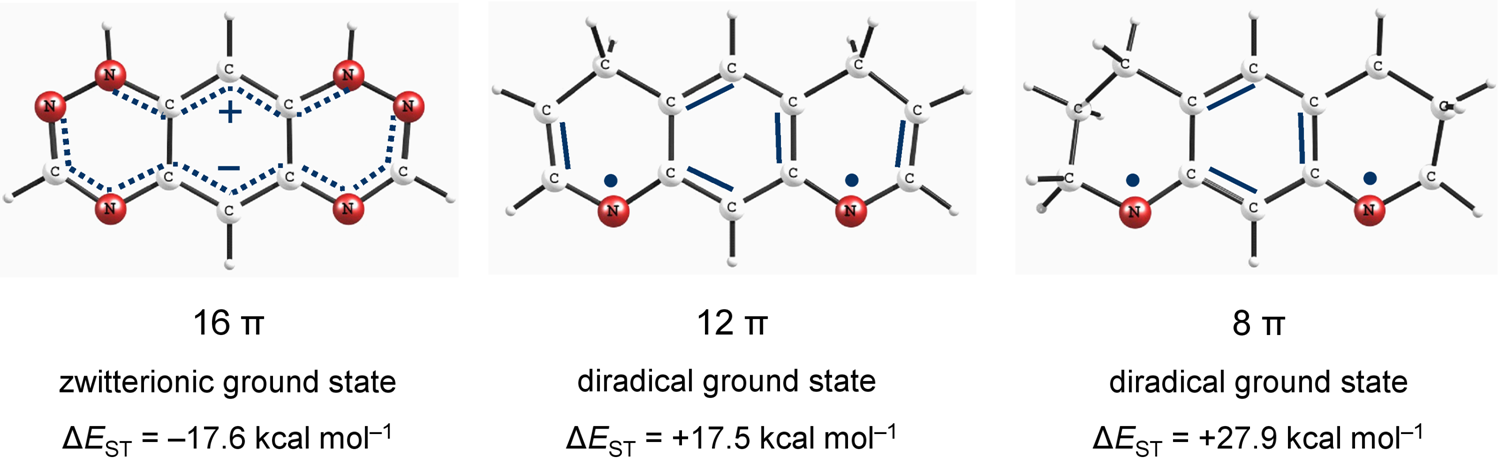
Non-Kekulé N-Substituted m-Phenylenes: N-Centered Diradicals versus Zwitterions. Shadi Amiri, Peter R. Schreiner* J. Phys. Chem. 2009, 113, 11750–11757. Download
Abstract. The relationship between the structures and electronic ground state properties of non-Kekulé-type N-substituted m-phenylenes was studied utilizing density functional theory (DFT), with the aim of determining the factors that lead to ground state triplet diradicals. At B3LYP/6-311G(d,p)//B3LYP/6-311G(d,p) we identified octahydropyrido-quinoline with an exceptionally large singlet–triplet energy separation of +27.9 kcal mol–1, in favor of the triplet. The high-spin structures can readily be obtained by interruption of the full cyclic π-delocalization that avoids cross-conjugation of the nitrogen radical centers. Introduction of additional heteroatoms on the other hand preferentially stabilizes the singlet zwitterionic resonance contributors in these systems. The identified diradicals show strong ferromagnetic exchange interactions between two radical-centers.
3,5,7,9-Substituted Hexaazaacridines: Toward Structures with Nearly Degenerate Singlet-Triplet Energy Separations. Peter Langer,* Shadi Amiri, Anja Bodtke, Nehad Saleh, Klaus Weisz, Halmar Görls, and Peter R. Schreiner*
J. Org. Chem. 2008, 73, 5048. Download
Abstract. Toward the goal of preparing stable, neutral open-shell systems, we synthesized a novel series of p-phenylsubstituted 3,5,7,9-hexaazaacridine and 3,5,7,9-hexaazaanthracene derivatives. The effects of substitution on the molecular electronic properties were probed both experimentally and computationally [B3LYP/6-311G(d,p)//B3LYP/6-31G(d,p)]. While the experimentally prepared structures already have small (20–25 kcal/mol) singlet-triplet energy gaps, systems with even smaller (<9 kcal/mol) singlet-triplet energy separations can be realized through systematic variation of the substituent numbers, types, and patterns. Hexaazaanthracenes show generally smaller singlet-triplet energy gaps than hexaazaacridines. Nitrogenbonded σ- and π-acceptor substituents that cause positive inductive and mesomeric effects as well as carbon-bonded σ-donor substituents make substituted hexaazaanthracenes promising candidates for purely organic high-spin systems.

