Theoretical prediction of affinities to cucurbiturils – the blind prediction HYDROPHOBE Challenge
Cucurbit[n]urils (CBn)1-5 are water-soluble macrocyclic host molecules of the molecular container type (Figure 1), which are able to encapsulate numerous organic guests inside their hydrophobic cavity.3 Binding of guest molecules to CBn is driven by a composite effect, which entails (i) the release of high-energy water from the cavity (nonclassical hydrophobic effect),6 (ii) the desolvation of the guests' and inner hosts' molecular surfaces (classical hydrophobic effect),6 (iii) ion-dipole interactions with the carbonyl-laced portals, and (iv) differential London dispersion interactions.7-11
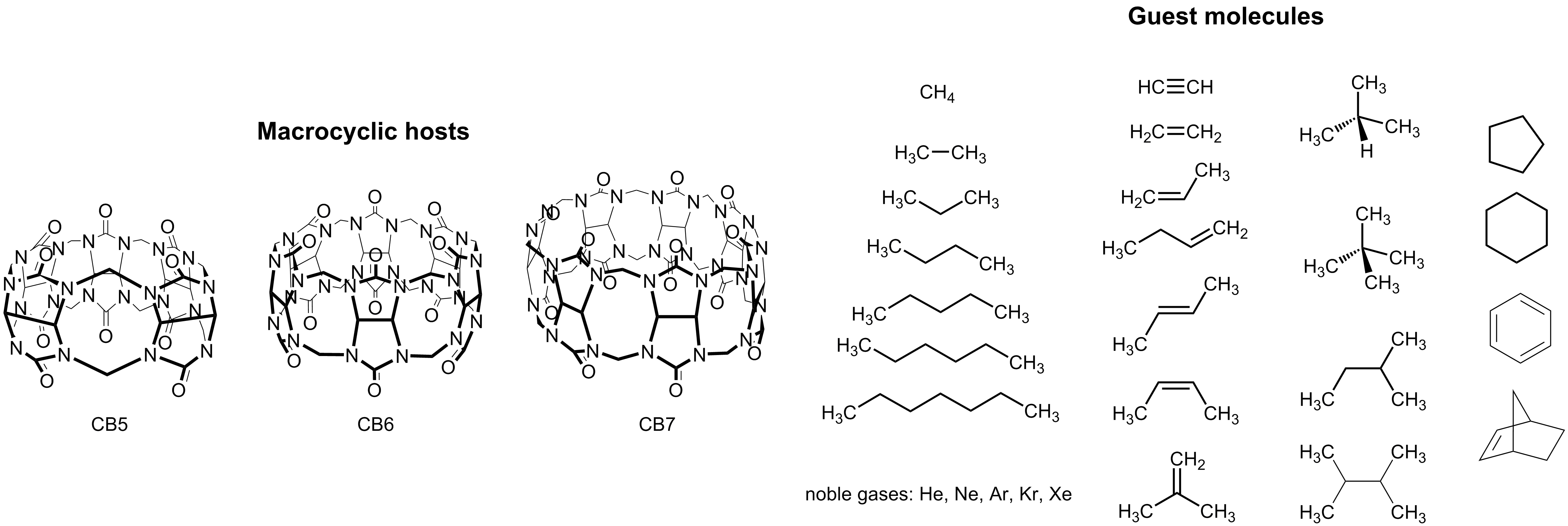
Figure 1. Chemical structures of CBn and suggested guest molecules for the blind prediction HYDROPHOBE challenge.
Through a dye-displacement strategy (which allows, among other, also the determination of binding constants with poorly water-soluble guests), the most comprehensive experimental data set of binding constants for neutral guests with cucurbiturils has now become available (Figure 1 and Table 1). It will be highly instructive to compare the data with theoretical predictions, considering only stoichiometric (1:1) complexes. As an advantage – and in contrast to previous recent challenges with macrocyclic hosts – the hosts and guests are consistently neutral and the experiments are carried out in salt-free water, which allows one (i) to neglect the type and concentration of (buffer) metal ions, whose presence modulates the experimental binding constants (a competitive binding effect which cannot be readily considered in the calculations of binding free energies) and (ii) to ignore counter ions of the guests (and hosts), the consideration of which introduces added complexity in the computational treatment.7, 9-16
We have a published data set of guest binding affinities available for CB6 (see Table 1),17 which can be used for referencing purposes. In addition, we call a blind prediction challenge for the prediction of binding affinities of hydrocarbons to CB7 (unpublished). Experimental binding constants of all noble gases to CB5 are presently under investigation and will also become available, such that computational predictions for noble-gas binding with CB5 and CB6 are also welcome. The series of hydrocarbons and noble gases allows both, a systematic variation in guest size (polarizability) and hydrophobicity without predominant ion-dipole interactions. Additionally, the comparison of CB6 and CB7 will expose the cavity size effect in terms of guest packing and the release of high-energy water from the cavity. In order to better understand affinity trends in aqueous solution and in order to conceptualize the outcome of the HYDROPHOBE challenge, the following additional questions – besides the prediction of the absolute affinities – should also be addressed:
-
Is there residual water inside the cucurbituril cavities, and how does it affect the free energies of guest binding in relation to any potential release of high-energy water?
-
How do the London dispersion interactions experienced by the guests inside the cavity compare to the London dispersion interactions of the guests immersed in water
Within the framework of the Priority Program “London Dispersion Interactions in Molecular Chemistry” (SPP 1807), we encourage scientists with experience in the computational prediction of supramolecular interactions, including those who have previously engaged in similar challenges such as the SAMPL4 challenge,9,13,15 those interested in the refined treatment of London dispersion interactions, and those working towards accurate theoretical corrections for aqueous solvent effects to participate.
Table 1. Binding constants of hydrocarbons with CB6, in water at pH 3.0, for ref. 17.
|
|
Hydrocarbon |
Ka/(103 M–1) |
|
Hydrocarbon |
Ka/(103 M–1) |
|
C1 |
methane |
<2 |
C4 |
trans-butene |
21 |
|
C2 |
ethane |
24 |
|
isobutane |
850 |
|
|
ethane |
3.9 |
|
isobutane-d10 |
910 |
|
|
acetylene |
0.11 |
|
isobutene |
84 |
|
C3 |
propane |
180 |
C5 |
n-pentane |
9 |
|
|
propane-d8 |
160 |
|
isopentane |
15 |
|
|
propene |
25 |
|
neopentane |
<2 |
|
C4 |
n-butane |
280 |
|
cyclopentane |
1300 |
|
|
1-butene |
79 |
|
higher alkanes |
<2 |
|
|
cis-butene |
150 |
|
|
|
If you wish to enter the challenge, please register with Dr. Khaleel Assaf (Email: k.assaf@jacobs-university.de), who will also be looking forward to receive your computational results prior to May 29th 2016. We are grateful to have Pablo Ballester (Institut Català d’Investigació Química, Tarragona, Spain) as an independent curator for the experimental CB7/hydrocarbon binding data.
1. J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem. Int. Ed., 2005, 44, 4844-4870.
2. E. Masson, X. X. Ling, R. Joseph, L. Kyeremeh-Mensah and X. Y. Lu, RSC Adv., 2012, 2, 1213-1247.
3. S. J. Barrow, S. Kasera, M. J. Rowland, J. del Barrio and O. A. Scherman, Chem. Rev., 2015, 115, 12320-12406.
4. D. Shetty, J. K. Khedkar, K. M. Park and K. Kim, Chem. Soc. Rev., 2015, 44, 8747-8761.
5. K. I. Assaf and W. M. Nau, Chem. Soc. Rev., 2015, 44, 394-418.
6. F. Biedermann, W. M. Nau and H.-J. Schneider, Angew. Chem. Int. Ed., 2014, 53, 11158-11171.
7. S. Grimme, Chem. Eur. J., 2012, 18, 9955-9964.
8. T. Risthaus and S. Grimme, J. Chem. Theory Comput., 2013, 9, 1580-1591.
9. R. Sure, J. Antony and S. Grimme, J. Phys. Chem. B, 2014, 118, 3431-3440.
10. J. Antony, R. Sure and S. Grimme, Chem. Commun., 2015, 51, 1764-1774.
11. R. Sure and S. Grimme, J. Chem. Theory Comput., 2015, 11, 3785-3801.
12. S. Moghaddam, C. Yang, M. Rekharsky, Y. H. Ko, K. Kim, Y. Inoue and M. K. Gilson, J. Am. Chem. Soc., 2011, 133, 3570-3581.
13. H. S. Muddana, C. D. Varnado, C. W. Bielawski, A. R. Urbach, L. Isaacs, M. T. Geballe and M. K. Gilson, J. Comput. Aid. Mol. Des., 2012, 26, 475-487.
14. M. Sundararajan, J. Phys. Chem. B, 2013, 117, 13409-13417.
15. H. S. Muddana, A. T. Fenley, D. L. Mobley and M. K. Gilson, J. Comput. Aid. Mol. Des., 2014, 28, 305-317.
16. A. T. Fenley, N. M. Henriksen, H. S. Muddana and M. K. Gilson, J. Chem. Theory Comput., 2014, 10, 4069-4078.
17. M. Florea and W. M. Nau, Angew. Chem. Int. Ed., 2011, 50, 9338-9342.
