Forschungsschwerpunkte
Von apl. Prof. Dr. Georgios Scheiner-Bobis
Sulfated Steroids in Reproduction
Overview
Dehydroepiandrosterone sulfate (DHEAS) is the most abundant circulating steroid. Its concentration in plasma is between 1.3 and 6.8 µM, which is about 200-fold higher than the plasma concentrations of dehydroepiandrosterone (DHEA) (7 - 31 nM). DHEAS is produced mainly in the adrenal zona reticularis. It is derived from DHEA, which is almost entirely converted to DHEAS by a sulfotransferase. The sulfated steroid is then secreted into the serum.
Sulfated steroids like DHEAS have long been considered to be physiologically inactive waste products of steroid hormone metabolism. Nevertheless, the identification of cytosolic steroid sulfatases able to hydrolyze the sulfate from the steran moiety prompted the new idea that sulfated steroids constitute a reservoir that upon desulfation can serve as precursors for the biosynthesis of other biologically active steroid hormones. In analogy, DHEAS has been considered to be a pro-androgen that has to be converted into testosterone or other steroid hormones in order to exert its biological activity.
Although DHEAS is produced not only in adrenal cortex and brain but also in gonads, surprisingly little is known about the effects of the steroid on the various cells of the reproductive system. Therefore, by using the Sertoli cell line TM4 and the spermatogenic cell line GC-2 as models, we address in our investigations the possibility of a hormone-like action of DHEAS by searching for signaling cascades that might be induced by the sulfated steroid. We further address a possible physiological significance of the unveiled signaling events by addressing their effects the expression of proteins that might be critical for male reproduction. Finally we undertake a first attempt to classify the DHEAS receptor within a group of known hormone receptors.
In summary, our investigations call into question the heretofore generally accepted idea of DHEAS being simply a pro-androgen and demonstrates for the first time that DHEAS acts as a steroid hormone on GC-2 and TM4 cells and triggers the activation of signaling cascades that reflects the non-classical signaling pathway of steroid hormones involving membrane-bound GPCRs that interact with the G-protein Gna11. The results of our investigations are consistent with the idea that DHEAS, by acting as an autonomous hormone on spermatogenic or Sertoli cells, may play a substantial role in the regulation of male fertility.
Our recent research:
A) Dehydroepiandrosterone sulfate mediates activation of transcription factors CREB and ATF-1 via a Gna11-coupled receptor in the spermatogenic cell line GC-2
DHEAS acting on the spermatogenic cell line GC-2 induces a time- and concentration-dependent phosphorylation of c-Src and Erk1/2 and activates the transcription factors ATF-1 and CREB. These actions are consistent with the non-classical signaling pathway of testosterone and suggest that DHEAS is a pro-androgen that is converted into testosterone in order to exert its biological activity. The fact, however, that steroid sulfatase mRNA was not detected in the GC-2 cells and the clear demonstration of DHEAS-induced activation of Erk1/2, ATF-1 and CREB after silencing the androgen receptor by siRNA clearly contradict this assumption and make it appear unlikely that DHEAS has to be converted in the cytosol into a different steroid in order to activate the kinases and transcription factors mentioned. Instead, it is likely that the DHEAS-induced signaling is mediated through the interaction of the steroid with a membrane-bound G-protein-coupled receptor, since silencing of Gna11 leads to the abolition of the DHEAS-induced stimulation of Erk1/2, ATF-1, and CREB. Further work for the identification of the DHEAS receptor and of target mRNAs whose expression is controlled by the activation of the CRE promoters through the transcription factors CREB and ATF-1 will help to define new roles of DHEAS in the physiology of cells of the male and possibly also of the female reproduction system.
B) Tight-junction dynamics of Sertoli cells are regulated by dehydroepiandrosterone sulfate interacting with a G-protein-coupled receptor.
Within a rather short timeframe of two hours nanomolar and low micromolar concentrations of DHEAS trigger in the Sertoli cell line TM4 the activation of Erk1/2, CREB, and ATF-1 that can be demonstrated by immunofluorescence experiments and western blots. This signaling cascade might be of physiological significance. Activation of Erk1/2 and of the transcription factor CREB (and possibly of ATF-1) is essential for spermatogenesis and maturation of spermatogonia to haploid spermatozoa and for the survival of spermatocytes. Little is known, however, whether and how activated CREB (or ATF-1) might directly influence the physiology of Sertoli cells, although its involvement in the regulation of Sertoli cell function has already been proposed but not further elaborated by others. To address the possibility of direct effects of DHEAS and CREB/ATF-1 activation on Sertoli cells we investigated the effects of the steroid on the expression of claudins. The rationale for this approach is based on the fact that expression of several claudins is also regulated by CRE promoters and that claudins, as constituents of tight-junctions (TJ), participate considerably in the formation and maintenance of the blood-testis barrier (BTB).
The BTB is one of the tightest blood-tissue barriers in mammals and separates the seminiferous epithelium into basal and adluminal compartments. The main function of the BTB is the formation of an immunological barrier in order to protect the meiotic and post-meiotic stages of the germ cells from cells of the immune system. Disturbance of the integrity of the BTB causes infertility.
Formation and maintenance of the BTB is mainly defined by the formation of TJ between neighboring Sertoli cells. TJ, in turn, are formed by the interactions of occludin with claudins, which interact with signalling proteins and proteins of the cytoskeleton on the cytosolic side of the membrane. With this information in mind, we focused the investigation of the physiological significance of DHEAS signaling on the effects of the hormone on claudin expression and the integrity of the BTB. Our results show for the first time that DHEAS stimulates the expression of claudin-3 and -5 at the mRNA/cDNA and protein levels and TJ formation between adjacent TM4 cells as evidenced by increased trans-epithelial resistance (TER). If one assumes that similar effects occur in vivo, these findings indicate that DHEAS may play a critical role in male fertility by regulating the formation and maintenance of the BTB. Since this sequence of events does not require the conversion of DHEAS to a desulfated form, one has to assume that a specific DHEAS receptor is directly involved in the signaling events. The question that remains, however, is what kind of receptor is it?
G-protein coupled receptors (GPCRs) have often been shown to trigger activation of Erk1/2 in numerous signaling cascades. In addition, various non-classical actions of steroid hormones are mediated through GPCRs. As mentioned above, DHEAS actions on the spermatogenic cell line GC2 is mediated through Gna11. Therefore we investigated a possible involvement of this protein in the DHEAS-induced signaling in TM4 cells. Immunofluorescence and western blot experiments demonstrated the importance of this protein, since silencing its expression by means of siRNA completely abolishes the DHEAS-induced activation of either Erk1/2 or the transcription factors CREB and ATF-1. Under these conditions the expression of TJ proteins claudin-3 and -5 and TJ formation in the presence of DHEAS never rises above the levels of their expression in the control cultures grown in absence of DHEAS, indicating the involvement of Gna11 and a membrane-bound GPCR in the stimulation of their expression.
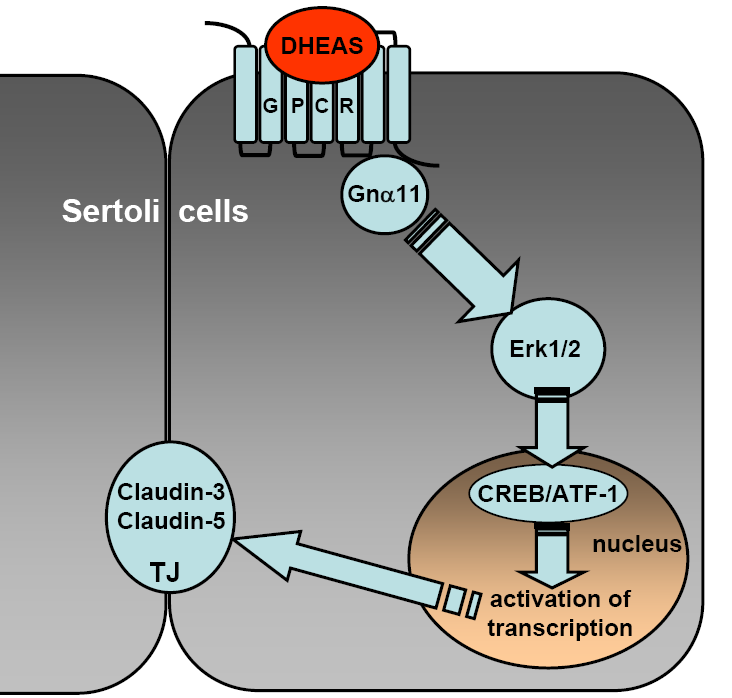
Fig. 1: Summary of the DHEAS-induced signaling events induced in the Sertoli cell line TM4. Interaction of DHEAS with a still undefined GPCR stimulates a signaling cascade responsible for the non-classical actions of steroid hormones. This signaling cascade is mediated by Gna11, which leads to Erk1/2 activation and to stimulation of the transcription factors CREB and ATF-1. Activated CREB and possibly ATF-1 stimulate the transcription of claudin-3- and claudin-5-specific mRNAs that are under the control of CRE promoters. As a result, claudin-3 and -5 protein expression and TJ formation between adjacent Sertoli cells are significantly increased.
Although this receptor has yet to be identified, the results of the current investigation (summarized in Figure 1) clearly point out that DHEAS acts as a hormone in its own right. By also taking into consideration the fact that DHEAS is produced in the gonads, one could expect an involvement of the steroid in the maintenance and dynamics of the BTB and therefore in the safeguarding of male fertility. Furthermore, since DHEAS is also produced in brain or adrenal cortex and claudin-3 or -5 are also constituents of various other blood-tissue barriers, it would not be unexpected if it were revealed that DHEAS influences the dynamics of these tissue-blood barriers as well. Thus, the extension of this investigation to other tissues and cell types might help to define new actions of DHEAS and establish its function as an essential steroid hormone in mammalian physiology.
This project is part of the DFG-Forschergruppe FOR1369, “Sulfated Steroids in Reproduction”. It is supported through DFG Sche 307/7-1.
Related publications:
[1] L. Konrad, R. Dietze, U. Kirch, H. Kirch, A. Eva, G. Scheiner-Bobis, Cardiotonic steroids trigger non-classical testosterone signaling in Sertoli cells via the a4 isoform of the sodium pump, Biochim. Biophys. Acta, 1813 (2011) 2118-2124.
[2] R. Dietze, L. Konrad, M. Shihan, U. Kirch, G. Scheiner-Bobis, Cardiac glycoside ouabain induces activation of ATF-1 and StAR expression by interacting with the a4 isoform of the sodium pump in Sertoli cells, Biochim. Biophys. Acta, 1833 (2013) 511-519.
[3] M. Shihan, U. Kirch, G. Scheiner-Bobis, Dehydroepiandrosterone sulfate mediates activation of transcription factors CREB and ATF-1 via a Ga11-coupled receptor in the spermatogenic cell line GC-2, Biochim. Biophys. Acta, 1833 (2013) 3064-3075..
[4] M. Shihan, A. Bulldan, G. Scheiner-Bobis, Non-classical testosterone signaling is mediated by a G-protein-coupled receptor interacting with Gna11, Biochim. Biophys. Acta, 1843 (2014) 1172-1181.
[5] R. Dietze, M. Shihan, A. Stammler, L. Konrad, G. Scheiner-Bobis, Cardiotonic steroid ouabain stimulates expression of blood-testis barrier proteins claudin-1 and -11 and formation of tight junctions in Sertoli cells, Mol. Cell. Endocrinol., 405 (2015) 1-13
[6] D. Papadopoulos, R. Dietze, M. Shihan, U. Kirch and G. Scheiner-Bobis, Tight-junction dynamics of Sertoli cells are regulated by dehydroepiandrosterone sulfate interacting with a G-protein-coupled receptor., Mol. Cell. Endocrinol, (2015; accepted).
Recent publications:
1. L. Karpova, A. Eva, U. Kirch, A. Boldyrev, G. Scheiner-Bobis.
Sodium pump a1 and a3 subunit isoforms mediate distinct responses to ouabain and are both essential for survival of human neuroblastoma. FEBS J (2010) 277, 1853-1860.
2. S. Ennen, S. Kloss, G. Scheiner-Bobis, K. Failing, A. Wehrend.
Histological, hormonal and biomolecular analysis of the pathogenesis of ovine Prolapsus vaginae ante partum. Theriogenology (2011) 75, 212-219.
3. G. Scheiner-Bobis.
The Na(+), K(+)-ATPase: more than just a sodium pump (Editorial).
Cardiovasc Res (2011) 89, 6-8
