Project Information
MECHANISMS OF MALE INFERTILITY
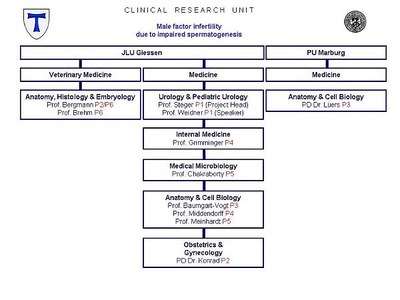
| APPLICANT |
Medical Faculty of the Justus Liebig University Giessen Department of Urology, Pediatric Urology and Andrology Speaker: Prof. Dr. med. Wolfgang Weidner Project Head: Prof. Dr. rer. nat. Klaus Steger |
 |
| PARTICIPATING FACULTIES |
Faculty of Biology of the Philipps University Marburg |
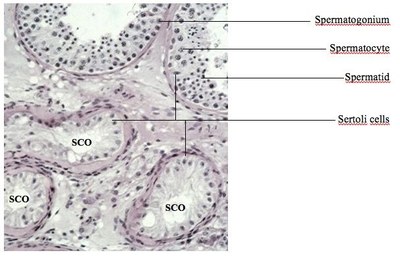
SPERMATOGENESIS occurs within the epithelium of the seminiferous tubules which contain somatic Sertoli cells and germ cells of differing developmental stages. Mitotic active spermatogonia both reproduce themselves for stem cell renewal and produce daughter cells that develop into spermatocytes. Spermatocytes undergo meiosis and give rise to spermatids which then transform into spermatozoa. This complex transformation, called spermiogenesis, includes nuclear condensation, acrosome formation and the development of a flagellum. Although the main developmental processes take place in the testis, the contribution of the epididymis to the maturation of spermatozoa is crucial. Beyond its role of storage and transport, it is during the sperm´s passage through the epididymis that progressive motility and fertilizing ability are attained.
As the testes from infertiliy men contain small areas with qualitatively normal spermatogenesis that allow testicular sperm extraction (TESE) to be carried out, therapeutic testicular biopsies play an important role in the treatment of their infertility. It is this diagnosis that the Clinical Research Unit KFO 181 seeks to improve by applying molecular and cell biology techniques. An outstanding feature of this Clinical Research Unit is the ability to follow up initial findings from animal and in-vitro model systems on cryopreserved and fixed human testicular material.
Testicular biopsies of these men reveal mixed atrophy with seminiferous tubules with qualitatively normal spermatogenesis directly adjacent to tubules with spermatogenic arrest and/or Sertoli-cell-only (SCO) (Figure).