Dr. Müller-Ruttloff, Christin
 |
Dr. Christin Müller-Ruttloff
|
|---|
Curriculum vitae
Tel: 0641-99-47751
Email: christin.mueller@viro.med.uni-giessen.de
Academic Career
| Since 06/2023 | Junior research group leader, Institute of Medical Virology |
| 05/2018-06/2023 | PostDoc, Institute of Medical Virology |
| 03/2013-05/2018 | PhD candidate, Institute of Medical Virology |
Academic distinctions
| 2022 | Von-Behring-Röntgen Nachwuchspreis |
| 2018 | 2nd Prize "PechaKucha" Contest, International Conference on Antiviral Research, Porto |
| 2018 | 2nd Prize for Best Young Investigator, Lipidomics Forum, Borstel |
| 2019-2017 | Three travel awards |
| 2015 | Poster Prize, Annual Meeting of German Society of Infectiology and German Center for Infection Research, Munich |
Funding
| 2024-2027 | Von Behring-Röntgen-Stiftung, Research Grant for Young Investigators, "Lipid architecture of CoV-induced replicative organelles." |
| 2024-2027 | DFG Sachbeihilfe "Role of lipid droplets in the formation of coronavirus-induced replicative organelles." |
| 2023-2026 | UKGM Research Grant for Young Investigators 06/2023 "In vitro characterization of antiviral aerosol therapy against respiratory viruses" |
| 2021-2024 | Early Career Support Grant, CRU309 Giessen, DFG |
Publications
ORCID: 0000-0002-6444-9478 (https://orcid.org/0000-0002-6444-9478)
Research focus
- Role of lipids and related enzymes in coronaviral replication
As intracellular parasites, viruses depend on the lipid metabolism of the infected host cell at each step of their replication cycle. In particular, viruses with a positive-sense, single-stranded RNA genome (+ssRNA viruses) induce extensive host membrane rearrangements, resulting in the formation of membranous microenvironments in the cytoplasm of infected cells. Inside these microenvironments, viral replication and transcription take place. Indeed, these so-called replicative organelles (ROs) are thought to provide a structural scaffold for the viral RNA synthesis machinery and contribute to sequestering viral components from host defence mechanisms, suggesting essential roles for ROs in viral replication. So far, the formation of coronavirus-induced membranous structures is poorly understood, and the specific roles of membrane biogenesis, lipids and associated cellular factors remain largely unknown. Exploring these aspects is, therefore, essential for understanding this process of RO formation from a molecular perspective.
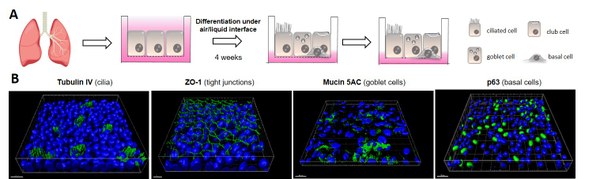
- In vitro aerosol therapy against respiratory viruses
Antiviral drug delivery by aerosol inhalation can provide several advantages over conventional therapies in combating respiratory viral infections. This includes (i) the avoidance of the liver-specific first-pass effect, (ii) a rapid onset of high local drug concentrations in the target tissue of respiratory pathogens, and (iii) minimal systemic side effects. Although aerosol inhalation is an attractive route, several factors, like drug formulation, stability, deposition, absorption, cytotoxicity, and antiviral efficacy, need to be considered. We aim to optimize these parameters in an ex vivo model for aerosolized drug delivery against respiratory infections. In detail, we mimic the human respiratory tract by using different (primary and permanent) airway epithelial cell culture models grown at an air-liquid interface, which are susceptible to viral infections. These cell models will then be combined with the vibrating-mesh nebulizer system ALICE-CLOUD (Air–Liquid Interface Cell Exposure–Cloud; Vitrocell®), allowing efficient in vitro aerosol application. This will help to understand whether and how nebulized antiviral compounds impair viral replication (especially SARS-CoV-2) in the airways for further development of inhalation therapy.