November
Bild des Monats November 2019
Hier finden Sie wechselnde Einblicke in die Arbeiten der am LaMa beteiligten Arbeitsgruppen. Eine Sammlung aller bisher erschienenen Bilder finden sie in der Galerie der Bilder des Monats.
Experimental assessment of the practical oxidative stability of solid electrolytes
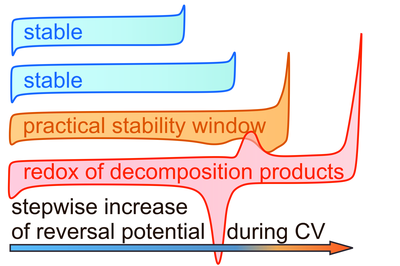
With the growing demand in safe and abundant energy storage systems, all-solid-state batteries are often expected to replace conventional lithium-ion batteries in the future. Among the variety of solid electrolytes for lithium-based batteries, especially lithium thiophosphates stand out due to promising ionic conductivities. However, degradation reactions at the electrode interfaces represent a major bottleneck for their application. Experimentally, stabilities of thiophosphates up to 5 V vs. Li/Li+ are often claimed from cyclic voltammetry (CV) using planar working electrodes. Contradictorily, redox activity of electrolytes was observed at lower potentials in solid-state batteries. The practical electrochemical and cycling stability of lithium thiophosphates are still critical issues that prevent long-term stable high-energy cells.
Using a stepwise increase in the reversal potentials in a CV experiment in combination with a high surface area carbon-electrolyte composite working electrode, the onset potential of oxidative decomposition, i.e. the oxidation of thiophosphate building units, can be identified. Furthermore, the results suggest that the crystalline solid electrolyte itself is not redox active, but rather that only after the formation of electrolyte decomposition products, significant redox behavior is observed. Indeed, the redox behavior of the decomposition products is an additional contributor to the overall cell capacity of solid-state batteries. The method serves as an efficient guideline for the determination of practical, kinetic stability limits of solid electrolytes with respect to the employed electrode materials.
G. Dewald et al. Chem. Mater. 2019, DOI: 10.1021/acs.chemmater.9b01550.
Dieses Bild wurde eingereicht von Georg Dewald, AG Dr. Wolfgang Zeier.