November
Bild des Monats November 2018
Hier finden Sie wechselnde Einblicke in die Arbeiten der am LaMa beteiligten Arbeitsgruppen. Eine Sammlung aller bisher erschienenen Bilder finden sie in der Galerie der Bilder des Monats.
Sodium storage behavior in graphite
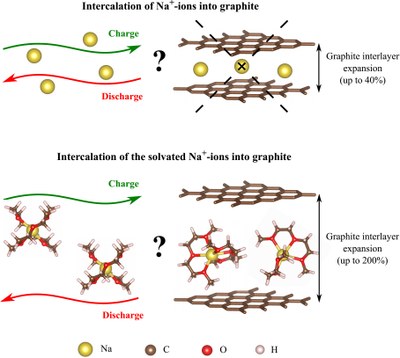
The development of energy storage systems has been a main focus of research in recent decades. Sodium-ion batteries (SIBs) have attracted great attention by offering more cost-efficient energy stores, which can be by achieved by replacing lithium by earth abundant and low cost sodium. One of the main drawbacks of SIBs remains the absence of thermodynamically stable sodium-rich graphite intercalation compounds (GICs). In the same time, rich binary GICs exist for other alkali metals, e.g. for lithium (LiC6), potassium (KC8), rubidium (RbC8) and cesium (CsC8). The contrast of Na intercalation behavior in graphite compared to other alkali metals raises a question “What is the origin of the instability of sodium-graphite intercalation compounds?” For this purpose, we examined the formation of Na-GICs by density functional theory (DFT) calculations and distinguish between the binding contributions and the structural deformation contributions.
Recently, a new approach of utilizing graphite as a negative electrode in SIBs was discovered. In fact, graphite is capable to intercalate solvated sodium ions, e.g. from ether-based electrolytes. Moreover, despite the large volume expansion of graphite, the co-intercalation reaction shows the excellent reversibility. With the group of Prof. Dr. Philipp Adelhelm from the Friedrich-Schiller-University in Jena we investigate the intercalation of solvated sodium ions into graphite. The main goal of our research is to gain a better understanding how the Na ions and solvent molecules are stored during the Na+-solvent co-intercalation into graphite, and to analyze the structure and thermodynamic stability of the resulting ternary GICs.
Dieses Bild wurde eingereicht von Dr. Olena Lenchuk, Arbeitsgruppe Prof. Dr. Doreen Mollenhauer.