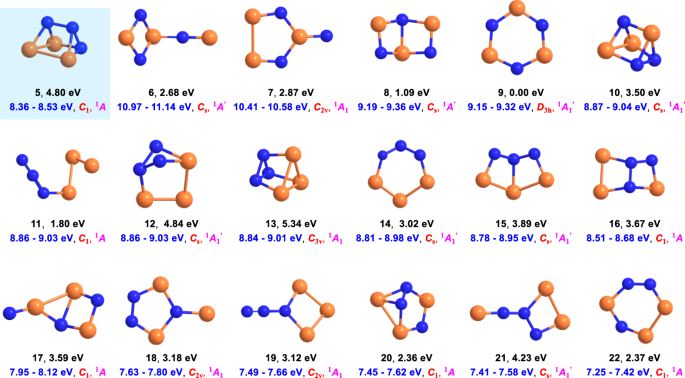
Identification of a prismatic P3N3 molecule formed from electron irradiated phosphine-nitrogen ices.
Cheng Zhu, André K. Eckhardt, Sankhabrata Chandra, Peter R. Schreiner, Ralf I. Kaiser
Nat. Commun. 2021, 12,5467. DOI: 10.1038/s41467-021-25775-1. Highlight: Featured in Chem. Eng. News 2021, 99(35), x.
Polyhedral nitrogen containing molecules such as prismatic P3N3 - a hitherto elusive isovalent species of prismane (C6H6) - have attracted particular attention from the theoretical, physical, and synthetic chemistry communities. Here we report on the preparation of prismatic P3N3 [1,2,3-triaza-4,5,6-triphosphatetracyclo[2.2.0.02,6.03,5]hexane] by exposing phosphine (PH3) and nitrogen (N2) ice mixtures to energetic electrons. Prismatic P3N3 was detected in the gas phase and discriminated from its isomers utilizing isomer selective, tunable soft photoionization reflectron time-of-flight mass spectrometry during sublimation of the ices along with an isomer-selective photochemical processing converting prismatic P3N3 to 1,2,4-triaza-3,5,6-triphosphabicyclo[2.2.0]hexa-2,5-diene (P3N3). In prismatic P3N3, the P–P, P–N, and N–N bonds are lengthened compared to those in, e.g., diphosphine (P2H4), di-anthracene stabilized phosphorus mononitride (PN), and hydrazine (N2H4), by typically 0.03–0.10 Å. These findings advance our fundamental understanding of the chemical bonding of poly-nitrogen and poly-phosphorus systems and reveal a versatile pathway to produce exotic, ring-strained cage molecules.