Generation and Reactivity of Phenylhydroxycarbenes in Solution.
Felix Keul, Artur Mardyukov and Peter R. Schreiner
J. Phys. Org. Chem. 2022, xx, yy–zz. in press. DOI: 10.1002/poc.4315.
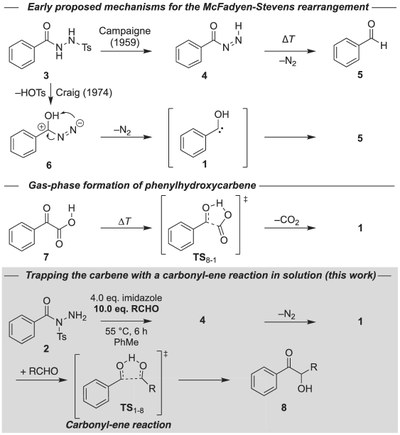
We provide evidence for the first successful generation of phenylhydroxycarbene and 4-trifluoromethylphenylhydroxycarbene in solution. The carbene tautomers of the corresponding benzaldehyde derivatives had been prepared under cryogenic matrix-isolation conditions before but their reactivity, apart from a prototypical quantum mechanical tunneling [1,2]-H-shift reaction, had not been studied. Here our strategy is to employ suitable carbene precursors for the McFadyen–Stevens reaction, to generate the parent and the para-CF3-substituted phenylhydroxycarbenes, and to react them with benzaldehyde or acetone in a highly facile, allowed six-electron carbonyl-ene reaction toward the corresponding α-hydroxy ketones. Our findings are supported by computations at the DLPNO-CCSD(T)/cc-pVQZ//B3LYP/def2-TZVP level of theory.