Über uns: AG Borggrefe

Transcriptional Regulation of Blood Cell Development
Unraveling the Molecular Mechanism of the Notch Signal Transduction Pathway
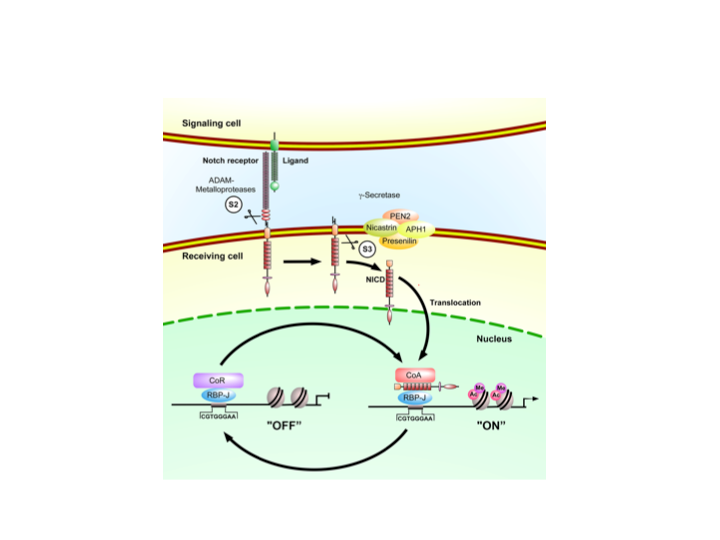
Notch signaling is an evolutionary conserved signal transduction pathway that plays pivotal roles in numerous aspects of cell differentiation, tissue homeostasis and tumorigenesis. In normal hematopoiesis Notch1 is essential for T-cell development and has been implicated in the maintenance of hematopoietic stem cells. At the molecular level, ligand binding induces the processing of the Notch transmembrane receptor resulting in the release of the intracellular domain of Notch (NICD), see also Figure 1. Subsequently, the NICD translocates to the nucleus, binds the transcription factor RBP-J and activates transcription of target genes. In the absence of the NICD, RBP-J actively represses Notch target genes through recruitment of corepressor complexes. My laboratory has extensively characterized this molecular switch (corepressor to coactivator) and characterized several Notch-cofactors (Oswald et al., 2005, Salat et al. 2008, Liefke et al. 2010, Wacker et al., 2010 and Jung et al., 2013).
Chromatin players in the Notch response:
Over the last few years we and other investigators have postulated that chromatin-based mechanisms play a key role in the control of Notch target gene expression (Borggrefe and Liefke, 2012). Some of the key questions are how histone methyltransferases and demethylases dynamically regulate histone methylation at specific target genes, how gene responsiveness is set up by activating and repressing methylation marks and how these enzymes work in concert with histone acetyltransferases (HATs), deacetylases (HDACs), kinases and phosphatases. We have for example demonstrated that Notch signaling dynamically regulates H3K4 methylation and showed that the H3K4 histone demethylase KDM5A plays a crucial role in this process (Liefke et al., 2010).
Characterization of the RBP-J repressor complex in leukemogenesis
Notch signaling have been implicated in the development of several forms of leukemia. We could identify ETO as part of the RBP-J corepressor complex. A chromosomal fusion protein AML1/ETO can disrupt the corepressor complex leading to a derepression of Notch target genes. We want to further characterize the underlying molecular mechanism that leads to leukemogenesis.
Characterization of the Notch-coactivator complex
Taking a biotinylation-tagging approach, we have isolated the RBP-J/Notch coactivator complex and characterized several of its components. For example, we have described the non-coding RNA Steroid-Responsive Activator (SRA) as part of the RBP/Notch coactivator complex. This complex is key in recruiting the acetyltransferase p300 that places the activating acetyl-marks on histones (Jung et al., 2013).
Posttranslational modifications (ubiquitinylation, phosphorylation, hydroxylation and acetylation) play a major role in the Notch response and tightly control the Notch coactivator turnover. Mutations in the C-terminal PEST-domain, that stabilize the Notch protein, have been detected in human T-ALL patients. In future, we will focus on the role of Notch modifying enzymes, which posttranslationally modify Notch itself, thereby controlling amplitude and duration of the Notch response.

