AG Fugmann

copyright: private
Prof. Dr. Tim Fugmann has studied technical Biology at the University of Stuttgart and later did his PhD at the ETH Zürich on "Innovative methodologies for the proteomic discovery of vascular markers in cancer and kidney disease". After the PhD he joined Philochem AG as head of target discovery with the aim to discover novel targets for pharmaceutical intervention. To better understand why certain patients respond to immunotherapy (which are developed by Philogen, the mother company of Philochem), his group implemented state-of-the-art HLA peptidomics technology for the study of T cell-cancer cell interactions. After nine years in industry, he moved to Berlin and joined the Max-Delbrück-Centrum for molecular medicine to work on bringing TCR T cell therapeutics from academic research into clinical trials. In February 2021 he was appointed to Professor for Proteomic with Focus on Mass Spectrometry at the JLU Giessen. The group worked with the state-of-the-art Orbitrap Fusion Eclipse mass spectrometer. In August 2022 Dr. Tim Fugmann left the Justus-Liebig-University and therefore our institute, too.
Research interests of the AG Fugmann:
1) Precision Medicine in lung cancer and autoimmune diseases
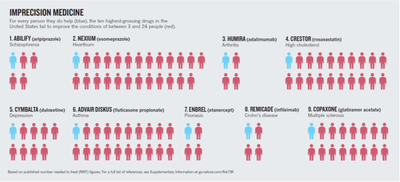
Irrespective of the high price tag associated with novel therapeutics, only around 10-40% of patients benefit from conventional therapy (see Figure 1). One of the reasons is that currently all patients with a certain (stage of) disease are treated with the same drug. However, as we know, each person has a unique genetic and epigenetic makeup resulting in variability of tolerated drug dose and response.
The solution is patient-centered treatment in which each patient receives the optimal therapy resulting in the best possible outcome. This will spare patients unnecessary treatments (and associated side effects) reducing patient burden, but also reduce healthcare costs (i.e. costs of ineffective drugs). Such patient-centered treatment requires precision medicine strategies, in which patients are diagnosed and classified precisely into a subgroup/phenotype which is associated with response to certain treatments.
While for few diseases, genetic and/or proteomic markers of patient subgroups are known (Her2-positive vs ER-positive vs triple negative breast cancer), for the majority of indications we are very far away from biomarker-directed treatment. The AG Fugmann aims to support the characterization of the individual patient with proteomic technologies to facilitate precision therapy of lung cancer and autoimmune diseases. Two technologies that are applied to facilitate precision medicine applications are outlined in 2) and 3).
copyright: private
Figure 1: The need for precision medicine. When treating every patient with the same drug (current strategy) only 4-24% of patients benefit from the treatment. In times in which therapies are getting more and more expensive, significant healthcare costs are associated with ineffective treatment. (adapted from Schork, N.J. (2015) Nature, 520, 611)
2) Characterizing the HLA-presented peptides in high resolution
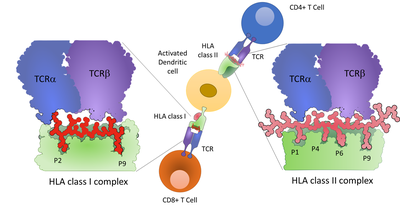
Adaptive immune processes are mediated by the interaction of T cells with the target cell through binding of the T cell receptor with peptides presented on HLA complexes (see Figure 2)
copyright: private
Figure 2: Presentation of peptides on HLA class I and HLA class II complexes on a dendritic cell. CD8+ T cells can interact with HLA class I molecules, while CD4+ T cells are interacting with HLA class II complexes. The molecular interaction of HLA, peptide and T cell receptor are shown, highlighting the binding register of the peptide to the HLA backbone.
With state-of-the-art methods and instruments, thousands of MHC-presented peptides can be identified after purifying the intact complexes with antibodies defining the immunopeptidome of tissues or cultured cells (MHC peptidomics; see Figure 2). The technology is established at the AG Fugmann and has previously been applied to murine organs, blood and cultured cells. The AG Fugmann is interested in developing more sensitive reproducible methodologies to profile the HLA peptidome more comprehensively, while facilitating the detection and quantification of disease relevant antigens.
copyright: private
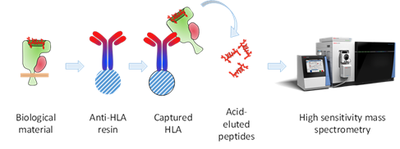
Figure 3. The process of identifying major histocompatibility antigen (MHC) [in humans called human leukocyte antigen or HLA]-presented peptides from cancer cells (HLA peptidomics). Cancer cells or tissue are lysed and HLA-peptide complexes are purified with high affinity anti-HLA antibodies. After stringent washing, the peptides are eluted in acid, further purified and finally subjected to high resolution mass spectrometry. With antibodies described in the literature, the different MHC complexes (Class I, Class II, mouse, man) can be purified (Purcell, T., et al. (2019) NatProtocols, 14, 1687).
3) Profiling the extracellular matrix and cell surface proteome to study pathologic processes
Especially in the tumor micro-environment, interactions between tumor cells, immune cells, fibroblasts and the extracellular matrix plays an important role in modulating the patient’s immune system facilitating tumor growth and resistance to therapy. On the other side, several therapeutic options target the tumor microenvironment and modulate the processes such as protease activity or are blocking anti-inflammatory mediators. For successful treatment of cancers, such as lung cancer, it is essential to understand the extracellular landscape of pro- and anti-inflammatory factors. The AG Fugmann has extensive experience in the proteomic characterization of extracellular matrix proteins and cell surface markers by in vivo, in vitro, ex vivo biotinylation and detergent-based fractionation. The results can be used to build systems level maps of cell types, proteases and immunmodulators guiding therapeutic decisions.
Publications can be found in PubMed.
Prof. Dr. Tim Fugmann
Science Tower
Feulgenstrasse 12
35392 Giessen
Tel.: +49 (0)641-985-46308
