AG PD Dr. Miray Rügen
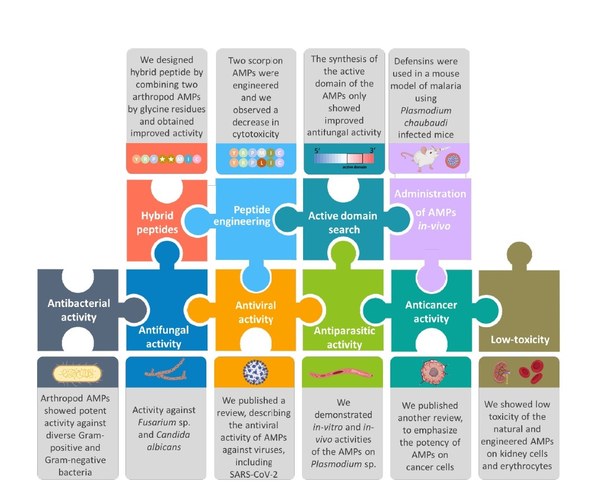
My group's primary focus is on the identification and functional characterization of arthropod AMPs. We examine those AMPs to demonstrate their activity against bacteria and fungi. Additionally, we conduct in vitro and in-vivo studies using Plasmodium falciparum in collaboration to find possible anti-malarial treatment options. Moreover, we engage in engineering AMPs to assess their impact on bacteria and murine cells. The latter cells are studied to evaluate the potential toxicity and side effects of the AMPs. Furthermore, we continually seek new AMP candidates to explore their medical potential in both human and veterinary medicine.
Selected research projects
Arthropod antimicrobial peptides (AMPs): from immunity to medical applications
Arthropods inhabit nearly every known environment and constantly encounter pathogens, compelling them to defend themselves with an effective immune system. Despite lacking antibodies or lymphocytes, they rely solely on their innate immunity, which includes both cellular and humoral responses. Among these responses, a notable feature is the rapid synthesis of antimicrobial peptides (AMPs), crucial in their innate immunity. These AMPs, derived from arthropods, exhibit remarkable efficacy against various pathogens, such as Gram-positive and Gram-negative bacteria, viruses, fungi, parasites, and even cancer cells. Consequently, natural AMPs are gaining attention as a promising new class of antimicrobials due to their extraordinary diversity and unique modes of action
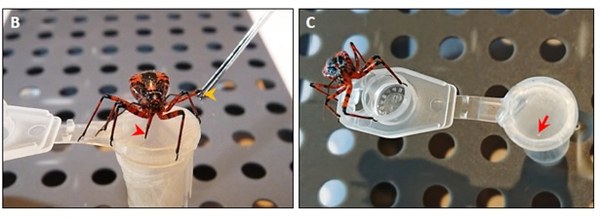
Venom composition and bioactivity profile from the Eurasian assassin bug Rhynocoris iracundus
Assassin bug venoms are potent and exert diverse biological functions, making them potential biomedical goldmines. Besides feeding functions on arthropods, assassin bugs also use their venom for defense purposes causing localized and systemic reactions in vertebrates. We use the venom from the assassin bug Rhynocoris iracundus and investigate its composition and bioactivity in vitro and in vivo. We showed that it caused lysis of murine neuroblastoma, hepatoma cells and healthy murine myoblasts. We demonstrated, for the first time, that assassin bug venom induces neurolysis and suggest that it counteracts paralysis locally via the destruction of neural networks, contributing to tissue digestion. The venom possesses specific antibacterial activity against some bacteria. We identified neurotoxic Ptu1, an ICK toxin homologous to ω-conotoxins from cone snails, cytolytic redulysins, and pore-forming hemolysins. We demonstrate the multifunctionality and complexity of assassin bug venom and currently looking for some interesting novel compounds as potential biotherapeutics.