Inhibition of hepatic fibrogenesis by matrix metalloproteinase-9 mutants in mice
The imbalance between matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases (TIMPs) is considered to be a pivotal parameter of deposition and breakdown of the extracellular matrix (1). TIMP-1, the most important endogenous inhibitor of most MMPs, plays a crucial role in the pathogenesis of liver fibrosis and may represent an important therapeutic target in the design of anti-fibrotic strategies for chronic liver disease (1-4). Previously we demonstrated that TIMP-1 is expressed in rat liver and regulated by inflammatory cytokines (5).

Effect of various cytokines on TIMP-1 mRNA levels in rat hepatocyte primary cultures (from Roeb et al. Hepatology 1993).
____________________________________________________________________________________
Furthermore, TIMP-1 is up-regulated by mouse oncostatin M, cardiotrophin-1, and transforming growth factor- b(TGF-
b) (6-9). Studies in cultured primary rat hepatocytes, hepatic stellate cells (HSC), and rat models of liver fibrosis, revealed an association of progressive fibrosis and increased TIMP-1 expression (5;10-12) .
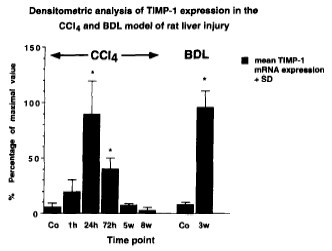
TIMP-1 expression in CCl4- and BDL induced liver injury (from Roeb et al. 1997 J. Hepatol.)
____________________________________________________________________________________
In human liver disease TIMP-1 expression is up-regulated five-fold in cirrhotic compared with normal liver and elevated TIMP-1 levels could be measured in plasma of such patients (13;14). Its expression occurred predominantly in areas of inflammation, in mesenchymal cells, hepatocytes, and endothelial cells (12;15). Chronic viral hepatitis B and C resulted in a significant increase in plasma TIMP-1 levels without elevation of TGF-b 1, a central mediator of fibrosis (16). Aminotransferase activity, a surrogate parameter for the inflammatory activity of viral hepatitis, correlated significantly with TIMP-1 expression. Thus, TIMP-1 plays a pivotal role in liver fibrosis. TIMP-1 plasma levels may prove useful as an early non-invasive marker of fibrosis, eventually suitable for the clinical management of chronic hepatitis (17). TIMP-1 overexpression does not result in liver fibrosis by itself, but strongly promotes liver fibrosis development (18).
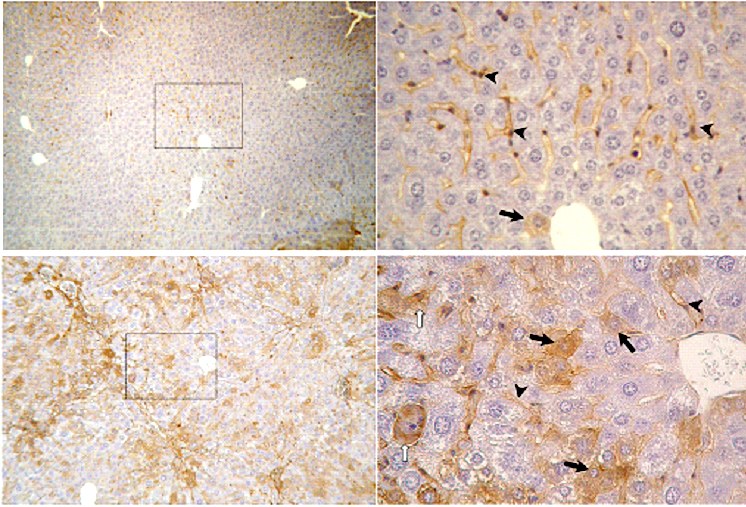
Immunhistochemical detection of TIMP-1 protein expression in murine liver sections. Upper panels: healthy control liver (i.p. oil treated BALB/c mouse), left: magnification 50x, low homogeneous distributed TIMP-1 expression; right: magn. 400x, TIMP-1 expression only in sinusoids (arrowheads), rarely in hepatocytes (arrow). Lower panels: fibrotic liver (i.p. CCl4 treated BALB/c mouse), left: magn. 100x, enhanced, inhomogeneous TIMP-1 expression predominantly in fibrotic areas (portal trias & septal areas); right: magn. 400x, sinusoidal expression of TIMP-1, enhanced TIMP-1 expression in hepatocytes (arrows).
____________________________________________________________________________________
Recently researchers have paid much attention to the reversal of hepatic fibrosis (3;19). Previous experiments demonstrated that TIMP-1 attenuates spontaneous resolution of liver fibrosis by the combination of a net reduction of the MMP activity and suppression of apoptosis in HSC (20). Murphy and co-workers demonstrated that TIMP-1 has anti-apoptotic effects in HSC, strongly depending on the level of MMP inhibition (21). They also demonstrated that persistent TIMP-1 expression in a model of carbon tetrachloride- (CCl4) induced cirrhosis is associated with persistence of HSC and decreased recovery of liver fibrosis. Recovery from comparatively advanced cirrhosis is possible and results in remodelling from micronodular cirrhosis to macronodular cirrhosis. Nevertheless, resolution of fibrosis is limited by a failure of HSC apoptosis (19). Increased TIMP-1 production in HSC and their central role in the pathogenesis of liver fibrosis may represent a promising therapeutic target in the design of anti-fibrotic strategies (2). Therefore, a specific neutralization of TIMP-1 would be beneficial. Many stimulators of TIMP-1 expression have been characterized, but little is known about blockade of TIMP-1 expression. Since the cloning of TIMP-1 in 1986, a reduction of TIMP-1 expression could only be demonstrated by dexamethasone (5) and concanavalin A (22).
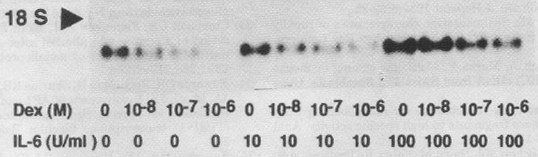
TIMP-1 mRNA stimulation with and without dexamethasone. Rat hepatocyte primary cultures were incubated for 8 hr with or without dexamethasone and IL-6 at different concentrations as indicated (from Roeb et al. Hepatology 1993).
____________________________________________________________________________________
Histological findings from patients with liver fibrosis and from animal models of fibrosis indicate that recovery from liver fibrosis with diminution of excess ECM proteins is possible (3;19;20). During recovery, an increased collagenase activity could be detected as a key mechanism in spontaneous resolution of fibrosis in rat liver homogenates (3). As fibrotic liver already exhibits an increased proteolytic activity, which is inhibited by high TIMP-1 activity, a possible therapeutic approach to interfere with fibrogenesis may be the specific neutralisation of TIMP-1. We have already shown that a catalytically inactive MMP-9 (MMP-9-H401A) that still binds TIMP-1 can be used as a specific antagonist of TIMP-1 activity in vitro (23).

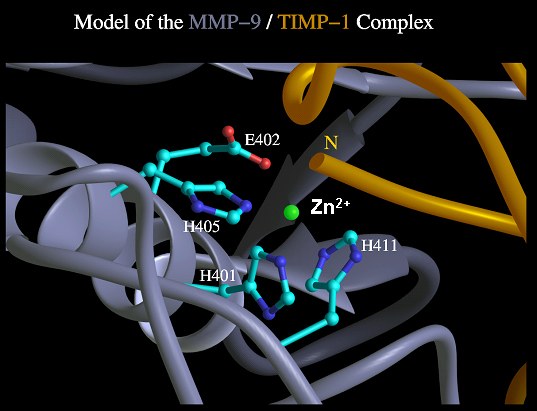
Molecular model of the MMP-9/TIMP-1 complex showing the active centre of MMP-9. The MMP-9 mutant H401A was created by an exchange of histidine 401 against alanine resulting in a loss of Zn2+ coordination. Glutaminic acid 402 has an essential function for the catalytic mechanism and was substituted by glutamine to build the second proteolytic inactive MMP-9 mutant. In the third mutant a slightly disturbed architecture of the catalytic centre was expected by the permutation of glutaminic acid 402 against histidine, and histidine 411 against glutaminic acid.
____________________________________________________________________________________
Recently different approaches to inhibit increased hepatic TIMP-1 levels were presented: Liu et al. investigated the effect of antisense TIMP-1 mRNA on activated HSC resulting in a decrease in collagen expression (24). Different alpha v beta 3-integrin antagonists preventing TIMP-1 mRNA expression were tested in HSC (25). Janoschek and co-workers utilized the TIMP-1 promotor in combination with the ganciclovir/thymidine kinase system for specific induction of programmed cell death in fibrogenic HSC (26) . Nevertheless, none of these approaches developed further from cell culture experiments. In generally our data agree with some recent paper (27;28). These results suffer from that either no approach on HSC biology was investigated or elucidated (e.g. apoptosis). We, however, could show that the antifibrotic effect of MMP-9 mutants was clearly caused by apoptosis of HSC.
The gene therapeutical application of MMP-1 and MMP-8 to induce fibrolysis (29;30) was discussed recently in the context of matrix degradation as a potential therapy for liver fibrosis (31). However, MMP activity is a crucial target in the therapy of cancer growth and metastasis (32) and in particular of hepatocellular carcinoma invasion (33). In contrast, we examined the TIMP-1 antagonistic effect of a proteolytically inactive MMP, apart from the fibrolytic properties of the wild type enzyme. Fibrotic liver exhibits increased proteolytic activity, which is inhibited by high TIMP-1 activity. Our therapeutical approach aimed towards the inactivation of TIMP-1.
We demonstrated that proteolytic inactive MMP-9 mutants, acting as TIMP-1 antagonists in vitro, inhibit CCl4 -induced liver fibrosis in mice (34).
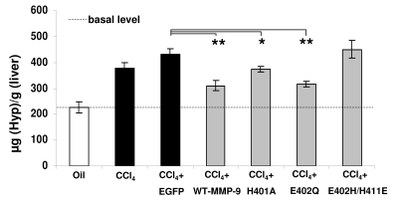
Reduction of ECM-deposition after treatment with MMP-9 mutants in CCl4 induced fibrosis. Quantification of hydroxyproline (Hyp) indicated different grades of fibrogenesis between controls (white and black bars) and the MMP-9 mutants treated mice (halftone bars).
____________________________________________________________________________________
Thus, we present a new and innovative therapy based on inactivated enzymes acting as scavengers for a strong promoter of liver fibrosis. The anti-fibrotic properties of MMP-9 mutants are based on their inhibitory effect on the transdifferentiation of HSC to the myofibroblast-like phenotype. Moreover, adenoviral application of some mutants (MMP-9-H401A and -E402Q) lead to an increased apoptosis of activated HSC in vitro and in vivo.
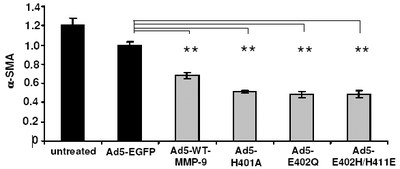
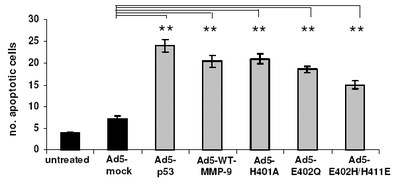
Influence of MMP-9 mutants on HSC transdifferentiation and apoptosis.
(a) The amounts of aSMA in HSC of control groups (black bars) and MMP-9 mutant treated groups (halftone bars) were measured densitometrically by Western blot analysis. Statistical significance is referred to the Ad-CMV-EGFP treated group only. (b) HSC treated as described under (a) The Annexin apoptosis assay revealed that apoptosis could be induced in plated HSC by treatment with TIMP-1 antagonists. The amount of apoptosis was quantified by counting Annexin-FITC-labelled cells at day five (randomly chosen fields of view). Statistical significance is referred to the Ad-CMV-sEGFP (mock transfection without expression) treated group.
____________________________________________________________________________________
The anti-fibrotic properties of MMP-9 mutants are based on their inhibitory effect on the transdifferentiation of HSC to the myofibroblast-like phenotype. Moreover, adenoviral application of some mutants (MMP-9-H401A and -E402Q) lead to an increased apoptosis of activated HSC in vitro and in vivo.
In summary our results suggest the following scenario of liver damage and protection by the MMP-9 mutants: CCl4 applied intraperitoneally is transported to the liver and is metabolized by hepatocytes (35). Subsequent cell damage or cell death results in the production of cytokines like TNF-a, TGF-b, IL-6, and IL-10, triggering the transdifferentiation process of HSC in paracrine and autocrine loops (36). Finally, activated HSC accelerate production of extracellular matrix (ECM), TIMPs, cytokines, and several MMPs promoting fibrogenesis of the liver (37). TIMP-1 was found to suppress apoptosis in activated HSC (20;21). However, if the level of free TIMP-1 is reduced by complex-formation with the MMP-9 mutants E402Q or H401A, apoptosis of activated HSC is induced. This decrease of activated HSC may then interrupt the fibrogenic process and the stimulation of HSC transdifferentiation. At this stage the inherited increased proteolytic activity in fibrotic liver becomes obvious and fibrolysis will be accelerated.
In vitro and in inbreed mice models we have shown that inactive MMP-9 mutants inhibit hepatic fibrosis by TIMP-1 interception. On the basis of the present study, we expect that the application of MMP-9 mutants as TIMP-1 scavengers will open a new avenue for therapeutic treatment of hepatic fibrosis.
References
1. Gomez, D. E., Alonso, D. F., Yoshiji, H., Thorgeirsson, U. P. (1997) Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur.J.Cell.Biol. 74, 111-122
2. Arthur, M. J., Mann, D. A., Iredale, J. P. (1998) Tissue inhibitors of metalloproteinases, hepatic stellate cells and liver fibrosis. J.Gastroenterol.Hepatol. 13 Suppl, 33-38
3. Iredale, J. P., Benyon, R. C., Pickering, J., McCullen, M., Northrop, M., Pawley, S., Hovell, C., Arthur, M. J. (1998) Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J.Clin.Invest. 102, 538-549
4. Bataller, R., Brenner, D. A. (2005) Liver fibrosis. J.Clin.Invest 115, 209-218
5. Roeb, E., Graeve, L., Hoffmann, R., Decker, K., Edwards, D. R., Heinrich, P. C. (1993) Regulation of tissue inhibitor of metalloproteinases-1 gene expression by cytokines and dexamethasone in rat hepatocyte primary cultures. Hepatology 18, 1437-1442
6. Richards, C. D., Kerr, C., Tanaka, M., Hara, T., Miyajima, A., Pennica, D., Botelho, F., Langdon, C. M. (1997) Regulation of tissue inhibitor of metalloproteinase-1 in fibroblasts and acute phase proteins in hepatocytes in vitro by mouse oncostatin M, cardiotrophin-1, and IL-6. J. Immunol. 159, 2431-2437
7. Peters, M., Roeb, E., Pennica, D., KH, M. z. B., Rose-John, S. (1995) A new hepatocyte stimulating factor: cardiotrophin-1 (CT-1). FEBS Lett. 372, 177-180
8. Kuyvenhoven, J. P., van Hoek, B., Blom, E., van Duijn, W., Hanemaaijer, R., Verheijen, J. H., Lamers, C. B., Verspaget, H. W. (2003) Assessment of the clinical significance of serum matrix metalloproteinases MMP-2 and MMP-9 in patients with various chronic liver diseases and hepatocellular carcinoma. Thromb Haemost. 89, 718-725
9. Kordula, T., Guttgemann, I., Rose-John, S., Roeb, E., Osthues, A., Tschesche, H., Koj, A., Heinrich, P. C., Graeve, L. (1992) Synthesis of tissue inhibitor of metalloproteinase-1 (TIMP-1) in human hepatoma cells (HepG2). Up-regulation by interleukin-6 and transforming growth factor beta 1. FEBS Lett. 313, 143-147
10. Roeb, E., Graeve, L., Mullberg, J., Matern, S., Rose-John, S. (1994) TIMP-1 protein expression is stimulated by IL-1 beta and IL-6 in primary rat hepatocytes. FEBS Lett. 349, 45-49
11. Iredale, J. P., Benyon, R. C., Arthur, M. J., Ferris, W. F., Alcolado, R., Winwood, P. J., Clark, N., Murphy, G. (1996) Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 24, 176-184
12. Roeb, E., Purucker, E., Breuer, B., Nguyen, H., Heinrich, P. C., Rose-John, S., Matern, S. (1997) TIMP expression in toxic and cholestatic liver injury in rat. J. Hepatol. 27, 535-544
13. Nie, Q. H., Cheng, Y. Q., Xie, Y. M., Zhou, Y. X., Cao, Y. Z. (2001) Inhibiting effect of antisense oligonucleotides phosphorthioate on gene expression of TIMP-1 in rat liver fibrosis. World J.Gastroenterol. 7, 363-369
14. Kasahara, A., Hayashi, N., Mochizuki, K., Oshita, M., Katayama, K., Kato, M., Masuzawa, M., Yoshihara, H., Naito, M., Miyamoto, T., Inoue, A., Asai, A., Hijioka, T., Fusamoto, H., Kamada, T. (1997) Circulating matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 as serum markers of fibrosis in patients with chronic hepatitis C. Relationship to interferon response. J. Hepatol. 26, 574-583
15. Herbst, H., Wege, T., Milani, S., Pellegrini, G., Orzechowski, H. D., Bechstein, W. O., Neuhaus, P., Gressner, A. M., Schuppan, D. (1997) Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am.J.Pathol. 150, 1647-1659
16. Gressner, A. M., Weiskirchen, R., Breitkopf, K., Dooley, S. (2002) Roles of TGF-beta in hepatic fibrosis. Front.Biosci. 7, d793-d807
17. Flisiak, R., Maxwell, P., Prokopowicz, D., Timms, P. M., Panasiuk, A. (2002) Plasma tissue inhibitor of metalloproteinases-1 and transforming growth factor beta 1--possible non-invasive biomarkers of hepatic fibrosis in patients with chronic B and C hepatitis. Hepatogastroenterology 49, 1369-1372
18. Yoshiji, H., Kuriyama, S., Miyamoto, Y., Thorgeirsson, U. P., Gomez, D. E., Kawata, M., Yoshii, J., Ikenaka, Y., Noguchi, R., Tsujinoue, H., Nakatani, T., Thorgeirsson, S. S., Fukui, H. (2000) Tissue inhibitor of metalloproteinases-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology 32, 1248-1254
19. Issa, R., Zhou, X., Constandinou, C. M., Fallowfield, J., Millward-Sadler, H., Gaca, M. D., Sands, E., Suliman, I., Trim, N., Knorr, A., Arthur, M. J., Benyon, R. C., Iredale, J. P. (2004) Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology 126, 1795-1808
20. Yoshiji, H., Kuriyama, S., Yoshii, J., Ikenaka, Y., Noguchi, R., Nakatani, T., Tsujinoue, H., Yanase, K., Namisaki, T., Imazu, H., Fukui, H. (2002) Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology 36, 850-860
21. Murphy, F. R., Issa, R., Zhou, X., Ratnarajah, S., Nagase, H., Arthur, M. J., Benyon, C., Iredale, J. P. (2002) Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J.Biol.Chem. 277, 11069-11076
22. Overall, C. M., Sodek, J. (1990) Concanavalin A produces a matrix-degradative phenotype in human fibroblasts. Induction and endogenous activation of collagenase, 72-kDa gelatinase, and Pump-1 is accompanied by the suppression of the tissue inhibitor of matrix metalloproteinases. J.Biol.Chem. 265, 21141-21151
23. Roeb, E., Behrmann, I., Grotzinger, J., Breuer, B., Matern, S. (2000) An MMP-9 mutant without gelatinolytic activity as a novel TIMP-1-antagonist. FASEB J. 14, 1671-1673
24. Liu, W. B., Yang, C. Q., Jiang, W., Wang, Y. Q., Guo, J. S., He, B. M., Wang, J. Y. (2003) Inhibition on the production of collagen type I, III of activated hepatic stellate cells by antisense TIMP-1 recombinant plasmid. World J.Gastroenterol. 9, 316-319
25. Zhou, X., Murphy, F. R., Gehdu, N., Zhang, J., Iredale, J. P., Benyon, R. C. (2004) Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J.Biol.Chem. 279, 23996-24006
26. Janoschek, N., van de Leur, E., Gressner, A. M., Weiskirchen, R. (2004) Induction of cell death in activated hepatic stellate cells by targeted gene expression of the thymidine kinase/ganciclovir system. Biochem.Biophys.Res.Commun. 316, 1107-1115
27. Jiang, W., Wang, J. Y., Yang, C. Q., Liu, W. B., Wang, Y. Q., He, B. M. (2005) Effects of a plasmid expressing antisense tissue inhibitor of metalloproteinase-1 on liver fibrosis in rats. Chin.Med.J. 118, 192-197
28. Parsons, C. J., Bradford, B. U., Pan, C. Q., Cheung, E., Schauer, M., Knorr, A., Krebs, B., Kraft, S., Zahn, S., Brocks, B., Feirt, N., Mei, B., Cho, M. S., Ramamoorthi, R., Roldan, G., Ng, P., Lum, P., Hirth-Dietrich, C., Tomkinson, A., Brenner, D. A. (2004) Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology 40, 1106-1115
29. Iimuro, Y., Nishio, T., Morimoto, T., Nitta, T., Stefanovic, B., Choi, S. K., Brenner, D. A., Yamaoka, Y. (2003) Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology 124, 445-458
30. Siller-Lopez, F., Sandoval, A., Salgado, S., Salazar, A., Bueno, M., Garcia, J., Vera, J., Galvez, J., Hernandez, I., Ramos, M., Aguilar-Cordova, E., Armendariz-Borunda, J. (2004) Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology 126, 1122-1133
31. Iredale, J. P. (2004) A cut above the rest? MMP-8 and liver fibrosis gene therapy. Gastroenterology 126, 1199-1201
32. Overall, C., Lopez-Otin, C. (2002) Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat.Rev.Cancer 2, 657-672
33. Miyoshi, A., Kitajima, Y., Sumi, K., Sato, K., Hagiwara, A., Koga, Y., Miyazaki, K. (2004) Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br.J.Cancer. 90, 1265-73
35. Weber, L. W., Boll, M., Stampfl, A. (2003) Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit.Rev.Toxicol. 33, 105-136
36. Friedman, S. L., Arthur, J. P. (2002) Reversing hepatic fibrosis. Sci.Med. 8, 194-205
37. Iredale, J. P., Goddard, S., Murphy, G., Benyon, R. C., Arthur, M. J. (1995) Tissue inhibitor of metalloproteinase-I and interstitial collagenase expression in autoimmune chronic active hepatitis and activated human hepatic lipocytes. Clin.Sci.(Lond). 89, 75-81
