Molecular interactions between RNA viruses and cellular transcripts
...we are investigating the how RNA viruses interact with the cellular molecular machinery during infection
Analysis of interactions of viral proteins with cellular RNAs – how RNA viruses alter RNA networks
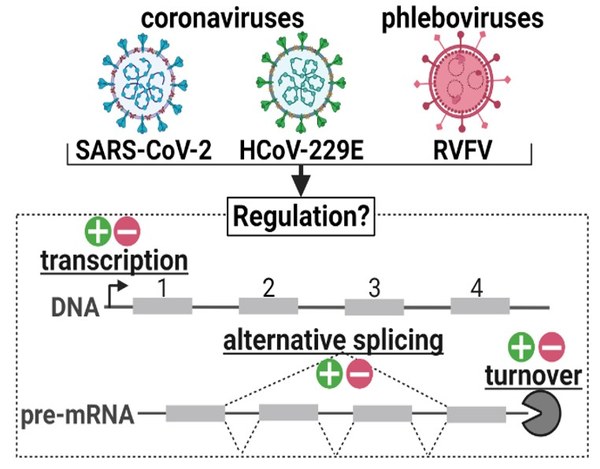
The molecular interactions between viral proteins and components inside of the host cell is an important and yet understudied field in RNA virology. The aim of our project is to systematically analyze the influence of RNA virus infection on the cellular mRNA life cycle across various virus families, and to identify interaction surfaces where therapeutic RNAs can interfere. We will first identify viral proteins that directly bind to RNAs and characterize their cellular RNA interaction partners using biochemical in vivo binding studies and sequencing (CLIP; UV-crosslinking and immunoprecipitation). Second, we will monitor changes in cellular mRNA processing and turnover induced by viral protein binding during infection, and third, we will develop and produce circRNA decoys that can interfere with these interactions, aimed at inhibiting crucial steps in the viral replication cycle. Research will focus on alpha- and beta-coronaviruses such as HCoV-229E and SARS-CoV-2 (positive-strand RNA viruses); and the Rift Valley Fever Virus (RVFV; a negative-strand Phlebovirus).
Functional analysis of interactions of the SARS-CoV-2 genome with microRNAs and virus inhibition by circular RNAs as a novel therapeutic concept
The molecular biology of the coronavirus SARS-CoV-2, the causative agent of the COVID-19 pandemic, still raises many questions. The cell surface receptors for virus entry are known, but a variety of intracellular conditions and factors has to be present for the virus to replicate efficiently and to produce a sufficiently high viral load to infect other cells, tissues or individuals. This explains why SARS-CoV-2 does not replicate in many tissues despite a suitable receptor set.
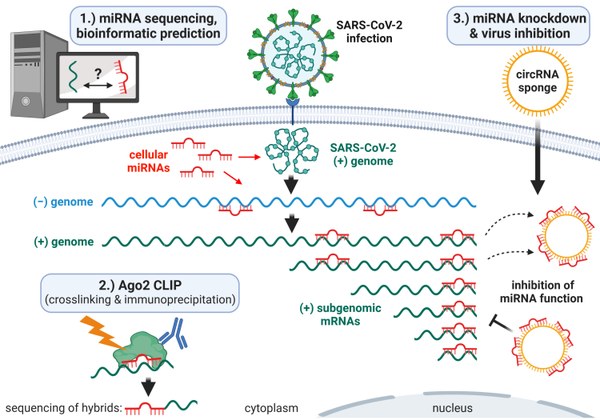
In this research project we plan to elucidate one of the mechanisms that contributes to tropism of SARS-CoV-2 and thereby unravel why it productively infects only certain cell types. We will investigate the interactions of the viral RNAs with microRNAs (miRNAs), and use this information to disrupt interactions essential for the virus in order to inhibit replication as basis for a novel therapeutic approach. MiRNAs are small cellular RNAs that naturally regulate the expression of proteins through binding to the corresponding mRNA. For other RNA viruses such as the Hepatitis C Virus (HCV) it was demonstrated that certain miRNAs are hijacked during viral infection. A direct interaction with the viral RNA is essential for HCV replication. SARS-CoV-2 is also a positive-strand RNA virus and its genome replicates in the cytoplasm and expresses a number of viral proteins. The presence of certain miRNAs is probably responsible for the fact that these processes can function efficiently.
To identify such miRNA-virus interactions, we will first determine the miRNA composition of certain cell types in which SARS-CoV-2 replicates efficiently by small RNA sequencing. Using this data set, we will then apply bioinformatic analyses to predict potential binding sites of abundant miRNAs on the SARS-CoV-2 genome. In parallel, the CLIP technology (crosslinking and immunoprecipitation) is used to experimentally determine miRNA/SARS-CoV-2 interactions. With CLIP, a certain protein is purified and all bound RNAs are identified by RNA-seq. In this case, CLIP will be performed in SARS-CoV-2 infected cells with the miRNA-associated protein Ago2. We can then purify and sequence complexes consisting of miRNAs and viral RNA, and thus monitor where on the virus genome and sub-genomic viral mRNAs the miRNAs bind and what function they might have. We will then specifically disrupt interactions that are essential for the SARS-CoV-2 replication cycle by inhibiting specific miRNAs using competing artificially produced circular RNA decoys. In a proof-of-principle study using HCV as a model system, we had already successfully validated this concept of virus inhibition as a novel antiviral therapeutic strategy. Mapping miRNA-virus interactions could support many other groups in the research of the molecular biology and pathogenesis of SARS-CoV-2 and provide the foundation for novel RNA-therapy approaches.