Birds as hosts of blood parasites
Prevalence and genetic diversity of avian malaria and avian malaria-like infections
Avian populations can be threatened by parasitic infections with avian Haemosporidia (Haemoproteus spp., Leucocytozoon spp., Plasmodium spp.)1. The haemosporidian prevalence in different bird taxa2, or in the same taxa in different regions3, can vary considerably. The parasites are transmitted by vectors as mosquitos(Plasmodium), biting midges (Haemoproteus and Leucocytozoon), and black flies (Leucocytozoon)2. Factors as habitat, species-specific behavior, host and vector density, and climate/‑changes could explain prevalence differences2. However, in contrast to quite a few herbivorous Psittaciformes species (parrots and cockatoos) that ingest antiparasitic secondary metabolites and did not harbor hemosporidian infections2, common German songbird species1 or columbid species, such as the endangered European Turtle Dove3, are often infected and thus may suffer fitness losses.
To detect the magnitude and genetic diversity of the haemosporidian infections of native species, their blood was examined by PCR for parasitic DNA (mitochondrial cytochrome b gene, ribosomal 18S RNA)1,3. An infection rate of 71% of adults with Leucocytozoon as well as two new Leucocytozoon-lineages and two new host-lineage associations were discovered in the songbirds examined1. The study of the six columbid species revealed a 42% infection rate with a total of 15 different haemosporidian lineages, with five lineages described for the first time3. Further research that also provides monitoring rationales for changes associated with global change is therefore needed3.
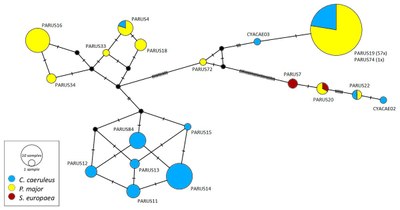
Fig. 1: Median-joining network of Leucocytozoon spp. The circles represent lineages, and the size of the circles is proportional to the frequencies of the lineages in the population. A hatch mark represents a mutation. The sampled host species are represented by different colors. The names of the lineages are noted at the corresponding circles1 (Fig.:3 S.11).
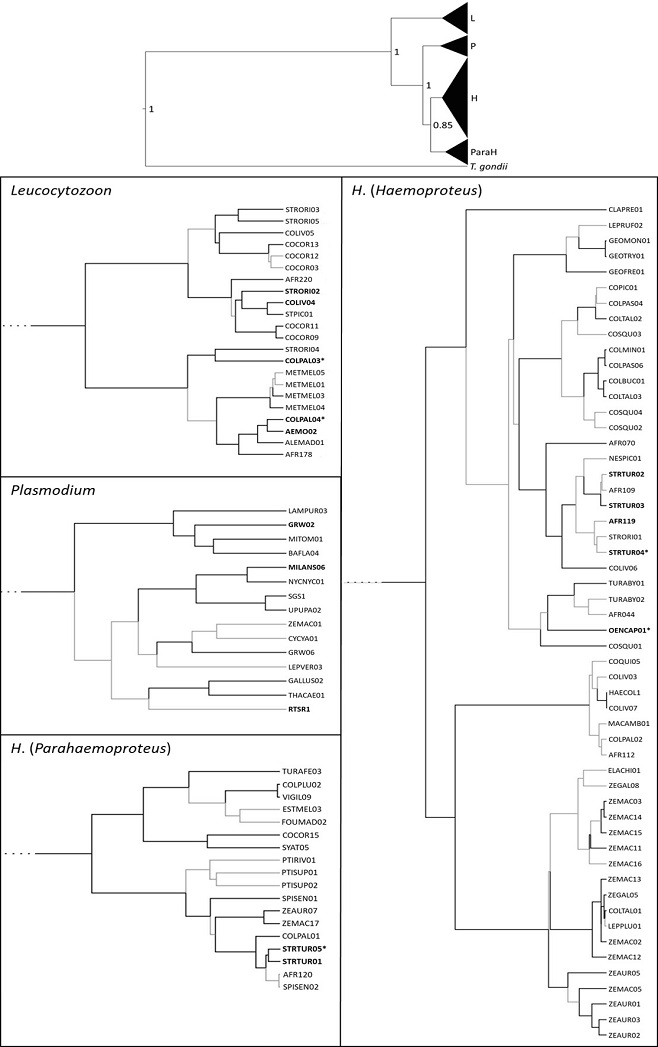
Fig. 2: Phylogeny of mitochondrial cytochrome b gene lineages of avian haemosporidian parasites isolated from blood samples of Columbiformes. The four different clades Leucocytozoon (L), Plasmodium (P), H. (Haemoproteus) (H) and H. (Parahaemoproteus) (ParaH) are shown in the overview tree and in one individual zoomed-in tree each together with lineage names. Lineages found in the present study are shown in bold. Newly discovered lineages are marked with ‘*’3 (Fig. 4 p. 1414).
References
1. Schumm, Y. R., Wecker, C., Marek, C., Wassmuth, M., Bentele, A., Willems, H., Reiner, G. & Quillfeldt, P. (2019). Blood parasites in Passeriformes in central Germany: prevalence and lineage diversity of Haemosporida (Haemoproteus, Plasmodium and Leucocytozoon) in six common songbirds. PeerJ, 6, e6259. https://peerj.com/articles/6259/
2. Masello, J. F., Martínez, J., Calderón, L., Wink, M., Quillfeldt, P., Sanz, V., Theuerkauf, J., Ortiz-Catedral, L., Berkunsky, I., Brunton, D., Díaz-Luque, J.A., Hauber, M.E., Ojeda, V., Barnaud, A., Casalins, L., Jackson, B., Mijares, A., Rosales, R., Seixas, G., Serafini P., Silva-Iturriza, A., Sipinski, E., Vásquez, R. A., Widmann, P., Widmann, I. & Merino, S. (2018). Can the intake of antiparasitic secondary metabolites explain the low prevalence of hemoparasites among wild Psittaciformes? Parasites & vectors, 11(1), 1-15.
https://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-018-2940-3
3. Schumm, Y. R., Bakaloudis, D., Barboutis, C., Cecere, J. G., Eraud, C., Fischer, D., Hering, J., Hillerich, K., Lormée, H., Mader, V., Masello, J.F., Metzger, B., Rocha, G., Spina, F. & Quillfeldt, P. (2021). Prevalence and genetic diversity of avian haemosporidian parasites in wild bird species of the order Columbiformes. Parasitology research, 120(4), 1405-1420.
https://link.springer.com/content/pdf/10.1007/s00436-021-07053-7.pdf
