October
Picture of the Month - October 2022
Increasing the available Discharge Capacity of Lithium Metal Anodes by incorporating a 3D-Network of Carbon Nanotubes for use in Solid-State Batteries
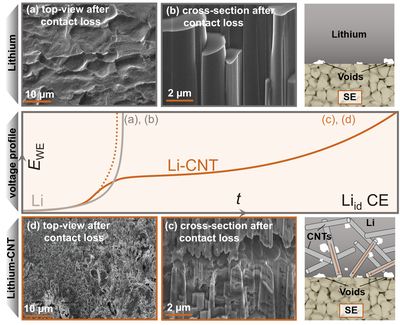
Employing lithium metal as the anode material in batteries would drastically enhance the cells energy density due to its highest possible specific capacity of 3860 mAh g-1. However, in combination with common liquid electrolytes, the formation of dendrites and detrimental degradation phases inhibit its implementation, which is why more chemically and mechanically resistive solid electrolytes may enable the use of lithium as the anode. However, a rigid solid electrolyte is not able to compensate pores that may arise at the interface during cell cycling, which is why modified lithium anode concepts may be needed. In this case, a composite was prepared via a melting route to incorporate 30 wt% of carbon nanotubes into lithium metal, which act as a three-dimensional host for the lithium with the ability to conduct both electrons and lithium atoms within the anode. As seen in the cryogenic (FIB)-SEM images, this means that while the dissolution during cell discharge only takes place at the direct, planar interface for pure lithium metal, it is expanded into the bulk of the electrode material via this carbon host. This can be seen as pores are formed also within the bulk of the electrode after cell discharge for the composites, whereas pure lithium metal is only dissolved at the direct interface to the solid electrolyte. This method therefore yields an anode discharge capacity of > 20 mAh cm-2 instead of only 1.2 mAh cm-2 for pure lithium metal and furthermore allows the tunability of the material’s mechanical properties. Further information can be found in a recent publication in Advanced Energy Materials (10.1002/aenm.202201125).
This picture was submitted by Till Fuchs, group of Prof. Dr. Jürgen Janek.
Further insights into the research activities of the ZfM groups can be found in the Gallery.