Research
Bidentate Lewis Acids Catalysis
Nature shows in every living system the power of catalysis. The high effectiveness and selectivity in Natures chemistry is without comparison. One trick Nature uses is the effect of multidentate binding, meaning that the substrates are hold in place by multiple interactions.
We learned from Nature by designing bidentate Lewis acids as new catalysts for Organic Synthesis. We illustrated the principle by developing the first catalyzed inverse electron-demand Diels-Alder (IEDDA) reaction of 1,2-diazines by a bidentate Lewis acid. We have also been able to incorporate such bidentate Lewis acid catalyzed IEDDA reactions in domino transformations.
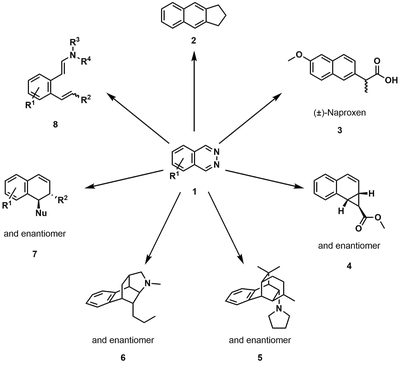
We also applied our method for the preparation of novel electrolytes, diazaquinones, for organic flow battery applications. Furthermore, we could extend the concept of bidentate catalysis to other processes, such as the activation of carbon dioxide CO2 or the release of hydrogen from ammonia borane.
Boron-Nitrogen materials
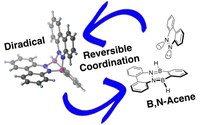
Based on the simultaneous interaction of bis-boron compounds with dinitrogen, we could access novel scaffold with unique properties, such as B,N-acenes or stable neutral diradicals.
Recent Publications
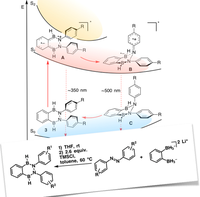
"Combining Bidentate Lewis Acid Catalysis and Photochemistry: Formal Insertion of o -Xylene into an Enamine Double Bond", S. Ahles, J. Ruhl, M. A. Strauss, H. A. Wegner, Org. Lett. , 2019 , 21, 11, 3927-3930 ; DOI: 10.1021/acs.orglett.9b01020 .
"Control of excited state conformation in B,N‐acenes", Z. Lu, H. Quanz, J. Ruhl, G. Albrecht, C. Logemann, D. Schlettwein, P. R. Schreiner, H. A. Wegne r, Angew . Chem. Int. Ed., 2019 , 58, 4259-4263; DOI: 10.1002/anie.201814104 .
