Imidazopyridine als neue Materialien
Imidazopyridines as new materials
Because of their biological and photophysical properties imidazo[1,5-a]pyridines 1 belong to an important class for both material chemistry and pharmaceutical industry. There are different technical applications for example OLEDs1 and FETs2 and different pharmaceutical applications for example as HIV-protease inhibitors3 and cardionic agents4.
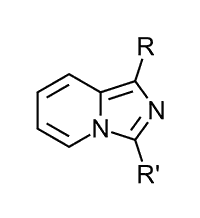
1
We are looking for a new, economical and efficient method to synthesize different imidazo[1,5-]pyridines. In the literature different methods are already known, but most of them depend on long reactions times or toxic reagents.
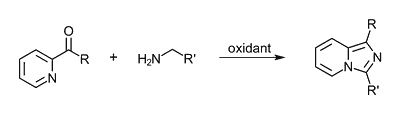
Scheme 1: Reaction of ketones and amines to imidazo[1,5-a]pyridines
At the same time the investigation of optical and electronical properties of imidazo[1,5-a]pyridines is another important field of our research. For that reason we have a close cooperation with the working group of Professor Derck Schlettwein (Institute of Applied Physics, University Giessen).
(1) Garino, C.; Ruiu, T.; Salassa, L.; Albertino, A.; Volpi, G.; Nervi, C.; Gobetto, R.; Hardcastle, K. I. Eur. J. Inorg. Chem. 2008, 2008, 3587–3591.
(2) H. Nakamura, H. Yamamoto, PCT Int. Appl. WO 2005043630; Chem. Abstr. 2005, 142, 440277.
(3) Kim, D.; Wang, L.; Hale, J. J.; Lynch, C. L.; Budhu, R. J.; Maccoss, M.; Mills, S. G.; Malkowitz, L.; Gould, S. L.; DeMartino, J. A.; Springer, M. S.; Hazuda, D.; Miller, M.; Kessler, J.; Hrin, R. C.; Carver, G.; Carella, A.; Henry, K.; Lineberger, J.; Schleif, W. A.; Emini, E. A. Bioorganic & medicinal chemistry letters 2005, 15, 2129–2134.
(4) Davey, D.; Erhardt, P. W.; Lumma, W. C.; Wiggins, J.; Sullivan, M.; Pang, D.; Cantor, E. J. Med. Chem. 1987, 30, 1337–1342.
| Project-Director: | Prof. Dr. Richard Göttlich |
|---|---|
| Co-Worker: | M.Sc. Jasmin Herr |
