Nanodiamonds (Diamondoids)
Diamondoids (nanometer-sized, hydrogen-terminated diamond hydrocarbons, nanodiamonds) are emerging as complementary materials to fullerenes and carbon nanotubes. In contrast to the latter, nanodiamonds are available in large quantities from crude oil, are chemically well-defined, and of high purity. Nanodiamonds are likely to share some of the unique properties of macroscopic diamond that are very attractive for a number of applications. One of the challenges with these novel building blocks is their selective and preparatively useful C–H bond functionalization. We have developed a variety of such approaches yielding numerous derivatives that can now be utilized for applications including platforms for organocatalysis and, in particular, organic molecular electronics.
- Directors: Univ.-Prof Peter R. Schreiner, Prof. Andrey A. Fokin
- Nanodiamonds (Diamondoids)
-
Current work:
Synthetic Doping of Diamondoids Through Skeletal Editing. Andrey A. Fokin, Olga K. Reshetilova, Vladislav V. Bakhonsky, Alexander E. Pashenko, Alena Kivernik, Tatyana S. Zhuk, Jonathan Becker, Jeremy E. P. Dahl, Robert M. K. Carlson and Peter R. Schreiner
Org. Lett. 2022, yy, xx. In press. DOI: 10.1021/acs.orglett.2c00982. Highlight: Selected as front cover picture of this issue.We present a strategy for the skeletal editing of diamondoid structures to selectively displace methylene for heteroatom moieties in the carbon framework. This constitutes a synthetic approach to doping diamond-like structures with electron donor dopants (O, N, and S). The key steps involve two subsequent retro-Barbier fragmentations followed by cage reconstruction in the presence of a dopant. Remarkably, the incorporation of n-dopants reduces the strain of the diamondoid cage as shown through homodesmotic equations.

The Role of Packing, Dispersion, Electrostatics and Solvation in High-Affinity Complexes of Cucurbit[n]urils with Uncharged Guests. Laura M. Grimm, Sebastian Spicher, Boryslav Tkachenko, Peter R. Schreiner, Stefan Grimme and Frank Biedermann
Chem. Eur. J. 2022, 28, xx–yy. accepted.Alkylphosphinites as Synthons for Stabilized Carbocations. Functionalized Nanodiamonds, part 89. Lukas Ochmann, Mika L. Kessler and Peter R. Schreiner
Org. Lett. 2022, 24,1460–1464. DOI: 10.1021/acs.orglett.2c00042.We present a new acid-free method for the generation of carbocations based on a redox condensation reaction that enables SN1 reactions with a variety of nucleophiles. We utilize readily synthesized phosphinites that are activated by diisopropyl azodicarboxylate to form betaine structures that collapse upon adding a pronucleophile, thereby yielding reactive carbocation intermediates. We also employ this approach for the alkylation of some bioactive molecules.

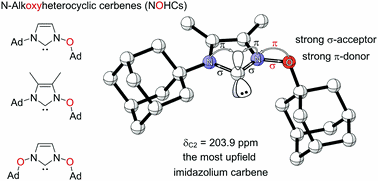
N-Alkoxyimidazolylidines (NOHCs): A new class of highly nucleophilic carbenes. Functionalized Nanodiamonds, part 88. Vladyslav V. Bakhonsky, Jonathan Becker, Grzegorz Mlostoń and Peter R. Schreiner
Chem. Commun. 2022, 58, 1538–1541. DOI: 10.1039/d1cc05696d.We report the first preparation of N-alkoxyimidazolylidene (NOHC), a nucleophilic carbene based on an oxidized imidazolium core. The Arduengo-type analogous carbene center shows the most upfield 13C NMR shift compared to common NHCs. The obtained gold(I) complex of the carbene follows the 13C NMR upfield trend and shows the marked influence the alkoxy substituents. Similarly, the 77Se and 15N NMR shifts of a range of NOHC-selenium adducts show increased σ-donation and decreased π-back donation in the bonding with the nucleophile. This extension of the NHC family provides altered electronic properties for the use of such carbenes as ligands or catalysts.
Regioselective synthesis of meta-tetraaryl substituted adamantane derivatives. Functionalized Nanodiamonds, part 87. Gowrisankar Saravanan, Bastian Bernhardt, Jonathan Becker and Peter R. Schreiner
Eur. J. Org. Chem. 2021, 6806–6810. DOI: 10.1002/ejoc.202101366.An easier path to organic white light emitters is accessible through the direct, meta-selective arylation of adamantane. The combination of AlCl3 and t-BuBr aids this reaction, which occurs smoothly, inter alia, also with fluorobenzene, which had proven to be difficult in the past and opposes the expected regioselectivity. Indeed, the products generate white light when irradiated with a continuous wave (CW) laser diode with improved intensities over the parent system, tetraphenyladamantane.

New meta-substituted tetraaryl adamantane derivatives were synthesized through one-step Friedel-Crafts adamantylation by using AlCl3 in combination with t-butyl bromide. The products exhibit improved (over tetraphenyl adamantane) highly directional white-light emission upon irradiation with a continuous wave (CW) laser diode.
Amorphous Molecular Materials for Directed Supercontinuum Generation. Functionalized Nanodiamonds, part 86. Stefanie Dehnen, Peter R. Schreiner, Sangam Chatterjee, Kerstin Volz, Nils W. Rosemann, Wolf-Christian Pilgrim, Doreen Mollenhauer and Simone Sanna
ChemPhotoChem 2021, 5, 1033–1041. DOI: 10.1002/cptc.202100130. Highlight: Cover picture of this issue and most accessed article in 2021.White-light generation: This Minireview summarizes current developments in the field of supercontinuum generation with amorphous molecular materials. The compounds in the focus of this work share a common general formula, [(RT)4E6] (T=C, Si, Ge, Sn; E=CH2, S; Se, Te), in which a molecular organic or inorganic adamantane-shaped cage is surrounded by four organic ligands R. All substances investigated in this context are described along with the results of experimental and theoretical analyses of their optical properties and the key parameters that influence the supercontinuum generation.

Molecular compounds of the general formula [(RT)4E6] (R=organic or organometallic substituent; T=C, Si, Ge, Sn; E=CH2, S, Se), hence adamantane derivatives and inorganic-organic hybrid compounds based on a heteroadamantane structure exhibit a non-linear optical response upon radiation with a continuous-wave near-infrared laser. The effect depends on the compounds’ habitus, which itself depends on the elemental composition of the cluster core, and on the nature of the organic substituents. A combination of these parameters that cause the material to be intrinsically amorphous leads to supercontinuum generation and thus to the emission of a broad spectrum, potentially appearing as white light. Notably, the emission essentially retains the driving laser's directionality. For crystalline samples, second harmonic generation is observed instead, which points to a close relationship of the optical properties and the intermolecular order. Variation of R, T, and E allows further fine-tuning of the emitted spectra. We present all studies made in regards to these effects and our overarching conclusions derived from them.
X-ray spectroscopic identification of strain and π-interactions in a series of saturated carbon-cage molecules: adamantane, twistane, octahedrane, and cubane. Trevor M. Willey, Jonathan R. I. Lee, Daniel Brehmer, Lasse Landt, Peter R. Schreiner, Andrey A. Fokin, Boryslav A. Tkachenko, Armin de Meijere, Sergei Kozhushkov, Anthony W. van Buuren
J. Vac. Sci. Technol. A 2021, 39, 053208. DOI: 10.1116/6.0001150.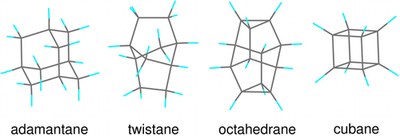 Novel nanocarbons such as fullerenes, nanotubes, graphene, and nanodiamond reside at the cutting edge of nanoscience and technology. Along with chemical functionalization, geometric constraints (such as extreme curvature in nanotubes or defects within or at the surfaces of diamond nanoparticles) significantly alter the electronic states of the nanocarbon material. Understanding the effects of steric strain on the electronic structure is critical to developing nanoelectronic applications based on these materials. This paper presents a fundamental study of how strain affects the electronic structure in a benchmark series of some fundamental saturated carbon cage compounds. Adamantane, C10H16, the smallest diamondoid and arguably the smallest nanodiamond crystallite, has carbon atoms essentially commensurate with diamond lattice positions and possesses by far the least molecular strain of this series. Twistane also is a C10H16 isomer but the fixed cyclohexane twist conformation of the central ring introduces additional strain into the cage. Octahedrane [(CH)12] and cubane [(CH)8] are considerably more strained, culminating in cubane where carbon–carbon bonds lie either parallel or orthogonal to one another. Using gas-phase near-edge x-ray absorption fine structure spectroscopy to probe the unoccupied electronic states, we observe two major progressions across this series. First, a broad C–C σ* resonance in the absorption splits into two more narrow and intense resonances with increasing strain. Second, the first manifold of states previously associated with tertiary C–H σ* in the diamondoid series appears to broaden and shift to lower energy. This feature is more than twice as intense in cubane than in octahedrane, even though these two molecules have only tertiary carbons, with the chemical formula (CH)x. The spectral differences are entirely due to the shape of the molecules; in particular, in cubane, the features arise from a high degree of p-p interaction between parallel C–C bonds. In contrast to the conventional wisdom that near-edge x-ray absorption is primarily an atomically localized spectroscopy, molecular shape and associated strain lead to the dominant features in spectra acquired from this fundamental series of carbon cage structures.
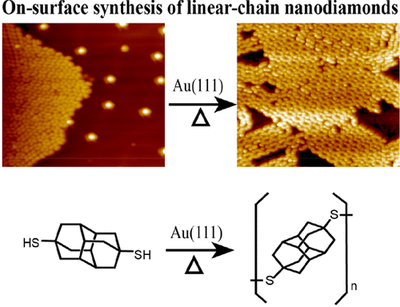
Novel nanocarbons such as fullerenes, nanotubes, graphene, and nanodiamond reside at the cutting edge of nanoscience and technology. Along with chemical functionalization, geometric constraints (such as extreme curvature in nanotubes or defects within or at the surfaces of diamond nanoparticles) significantly alter the electronic states of the nanocarbon material. Understanding the effects of steric strain on the electronic structure is critical to developing nanoelectronic applications based on these materials. This paper presents a fundamental study of how strain affects the electronic structure in a benchmark series of some fundamental saturated carbon cage compounds. Adamantane, C10H16, the smallest diamondoid and arguably the smallest nanodiamond crystallite, has carbon atoms essentially commensurate with diamond lattice positions and possesses by far the least molecular strain of this series. Twistane also is a C10H16 isomer but the fixed cyclohexane twist conformation of the central ring introduces additional strain into the cage. Octahedrane [(CH)12] and cubane [(CH)8] are considerably more strained, culminating in cubane where carbon–carbon bonds lie either parallel or orthogonal to one another. Using gas-phase near-edge x-ray absorption fine structure spectroscopy to probe the unoccupied electronic states, we observe two major progressions across this series. First, a broad C–C σ* resonance in the absorption splits into two more narrow and intense resonances with increasing strain. Second, the first manifold of states previously associated with tertiary C–H σ* in the diamondoid series appears to broaden and shift to lower energy. This feature is more than twice as intense in cubane than in octahedrane, even though these two molecules have only tertiary carbons, with the chemical formula (CH)x. The spectral differences are entirely due to the shape of the molecules; in particular, in cubane, the features arise from a high degree of p-p interaction between parallel C–C bonds. In contrast to the conventional wisdom that near-edge x-ray absorption is primarily an atomically localized spectroscopy, molecular shape and associated strain lead to the dominant features in spectra acquired from this fundamental series of carbon cage structures.Diamantane thiols on Metal Surfaces: Spatial Configurations, Bond dissociation, and Polymerization. Functionalized Nanodiamonds, part 85. Jun Feng, Ephrath Solel, Peter R. Schreiner, Harald Fuchs and Hong-Ying Gao
J. Phys. Chem. Lett. 2021, 12, 3468–3475. DOI: 10.1021/acs.jpclett.1c00387We report the on-surface chemistry of diamantanethiols on metal surfaces by combining low-temperature STM studies with quantum mechanical density functional theory computations. First, we examined the spatial configurations of diamantanethiols on metal surfaces, in which the thiol-substrate confinement plays a key role. We then thermally desorbed the diamantanethiols from the substrate surfaces to determine whether the C–S or S–metal bonds preferentially break. Finally, we explored diamantane-4,9-dithiol and its polymerization on metal surfaces, forming linear nanodiamond disulfur chains. This work broadens the fundamental knowledge of functionalized diamondoid behavior on surfaces and provides a novel approach to link diamantane as necklace-chain nanodiamond hybrid materials.
Chemical Probes for Blocking of Influenza A M2 WT and S31N Channels. Functionalized Nanodiamonds, part 83. Christina Tzitzoglaki, Kelly McGuire, Panagiotis Lagarias, Athina Konstantinidi, Anja Hoffmann, Nataliya Fokina, Chulong Ma, Ioannis P. Papanastasiou, Peter R. Schreiner, Santiago Vazquez, Jun Wang, Michaela Schmidtke, David D. Busath and Antonios Kolocouris
ACS Chem. Biol. 2020, 15, 2331–2337. DOI: 10.1021/acschembio.0c00553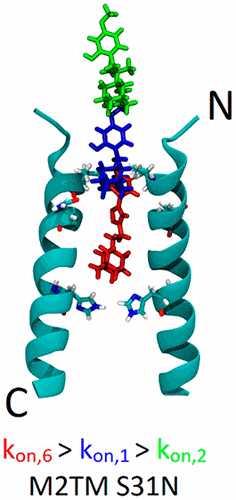
Synthesis and antiproliferative activity of hindered, chiral 1,2-diaminodiamantane platinum(II) complexes. Functionalized Nanodiamonds, part 82. Vladyslav V. Bakhonsky, Aleksander A. Pashenko, Jonathan Becker, Heike Hausmann, Huub J. M. De Groot, Herman S. Overkleeft, Andrey A. Fokin and Peter R. Schreiner
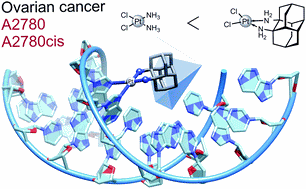
Dalton Trans. 2020, 49, 14009–14016. DOI: 10.1039/D0DT02391DPlatinum-based antineoplastic agents play a major role in the treatment of numerous types of cancer. A new bulky, lipophilic, and chiral ligand based on 1,2-diaminodiamantane in both of its enantiomeric forms was employed for the preparation of new platinum(II) complexes with chloride and oxalate ligands. The dichloride complexes have a higher solubility and were evaluated as anti-proliferation agents for human ovarian cancer cell lines A2780 and cisplatin-resistant A2780cis. Its R,R-enantiomer showed increased efficacy compared to cisplatin for both cancer cell lines. A chromatographic approach was used to estimate the solvent partition coefficient of the dichloride complex. The binding of diamondoid-based platinum complexes to nucleotides was tested for both enantiomers with guanosine monophosphate (GMP) and deoxyguanosine monophosphate (dGMP) and occurs at a similar or faster rate for both isomers compared to cisplatin despite greatly increased steric demand. These findings highlight the potential in 1,2-diaminodiamantane as a viable pharmacophore.
Incorporating Diamondoids as Electrolyte Additive into a Sodium Metal Anode to Mitigate Dendrite Growth. Functionalized Nanodiamonds, part 83. Julian J. A. Kreissl, Daniel Langsdorf, Boryslav A. Tkachenko, Peter R. Schreiner, Jürgen Janek, Daniel Schröder
ChemSusChem 2020, 13, 2661–2670. DOI: 10.1002/cssc.201903499
Owing to the high abundance and gravimetric capacity (1165.78 mAh g−1) of pure sodium, it is considered as a promising candidate for the anode of next‐generation batteries. However, one major challenge needs to be solved before commercializing the sodium metal anode: The growth of dendrites during metal plating. One possibility to address this challenge is to use additives in the electrolyte to form a protective solid electrolyte interphase on the anode surface. In this work, we introduce a diamondoid‐based additive, which is incorporated into the anode to target this problem. Combining operando and ex situ experiments (electrochemical impedance spectroscopy, optical characterization, and cycling experiments), we show that molecular diamondoids are incorporated into the anode during cycling and successfully mitigate the growth of dendrites. Furthermore, we demonstrate the positive effect of the additive on the operation of sodium‐oxygen batteries by means of increased energy density.
Vibrational signatures of diamondoid dimers with large intramolecular London dispersion interactions. Functionalized Nanodiamonds, part 81. Christoph Tyborski, Tobias Hückstaedt, Roland Gillen, Tommy Otto, Nataliya A. Fokina, Andrey A. Fokin, Peter R. Schreiner and Janina Maultzsch
Carbon 2019, 157, 201–207. DOI: 10.1016/j.carbon.2019.10.014We analyze the vibrational properties of diamondoid compounds via Raman spectroscopy. The compounds are interconnected with carbon-carbon single bonds that exhibit exceptionally large bond lengths up to 1.71 Å. Attractive dispersion interactions caused by well-aligned intramolecular H···H contact surfaces determine the overall structures of the diamondoid derivatives. The strong van-der-Waals interactions alter the vibrational properties of the compounds in comparison to pristine diamondoids. Supported by dispersion-corrected density functional theory (DFT) computations, we analyze and explain their experimental Raman spectra with respect to unfunctionalized diamondoids. We find a new set of dispersion-induced vibrational modes comprising characteristic CH/CH2 vibrations with exceptionally high energies. Further, we find structure-induced dimer modes that are indicative of the size of the dimers.
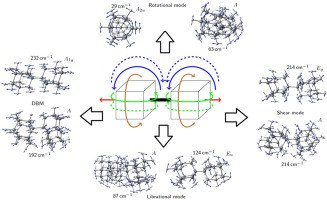
Structures and Dynamics in Thiolated Diamantane Derivative Monolayers. Functionalized Nanodiamonds, part 80. Yaroslava Yu. Lopatina, Viktoriya I. Vorobyova, Andrey A. Fokin, Peter R. Schreiner, Alexandr A. Marchenko, Tatiana S. Zhuk
J. Phys. Chem. C 2019, 123, 27477–27482. DOI: 10.1021/acs.jpcc.9b06625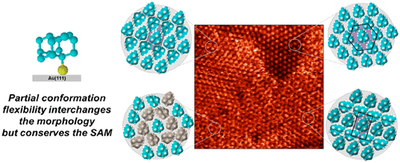
Self-assembled monolayers (SAMs) of isomeric 1-diamantantyl thiol (1SH) and 4-diamantantyl thiol (4SH) on Au(111) surfaces were characterized using scanning tunneling microscopy (STM) in air. The 4SH molecules form a hexagonal (√7 × √7)R19° structure. In monolayers of 1SH, two hexagonal packings, namely,
 and a more dense (
and a more dense ( structure, modulated by rectangular and double row structures were observed. The superstructures correspond to different metastable monolayer states due to intramolecular rotational degrees of freedom. The observed tip-induced structural polymorph changes show that SAMs of different morphologies coexist and may be transformed into each other. Thus, rigid hydrocarbon cages viewed as isotropic dispersion energy donors aggregate effectively within the given unit cell in various relative orientations with only little influence on the packing densities and SAM morphologies.
structure, modulated by rectangular and double row structures were observed. The superstructures correspond to different metastable monolayer states due to intramolecular rotational degrees of freedom. The observed tip-induced structural polymorph changes show that SAMs of different morphologies coexist and may be transformed into each other. Thus, rigid hydrocarbon cages viewed as isotropic dispersion energy donors aggregate effectively within the given unit cell in various relative orientations with only little influence on the packing densities and SAM morphologies.Diamondoid Nanostructures as sp3-Carbon-Based NO2 Sensors. Functionalized Nanodiamonds, part 79. Oana Moncea, Juan Casanova-Chafer, Didier Poinsot, Lukas Ochmann, Clève D. Mboyi, Eduard Llobet, Imen Makni, Molka El Atrous, Stéphane Brandès, Yoann Rousselin, Bruno Domenichini, Nicolas Nuns, Andrey A. Fokin, Peter R. Schreiner and Jean-Cyrille Hierso
Angew. Chem. Int. Ed. 2019, 58, 9933–9938. DOI: 10.1002/anie.201903089
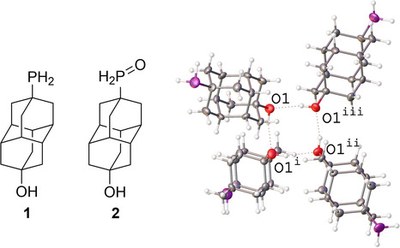
Highlighted in ChemistryViews.Diamondoids, sp3‐hybridized nanometer‐sized diamond‐like hydrocarbons (nanodiamonds), difunctionalized with hydroxy and primary phosphine oxide groups, enable the assembly of the first sp3‐C‐based chemical sensors by vapor deposition. Both pristine nanodiamonds and palladium nanolayered composites can be used to detect toxic NO2 and NH3 gases. This carbon‐based gas sensor technology allows reversible NO2 detection down to 50 ppb and NH3 detection at 25–100 ppm concentration with fast response and recovery processes at 100 °C. Reversible gas adsorption and detection is compatible with 50 % humidity conditions. Semiconducting p‐type sensing properties are achieved from devices based on primary phosphine–diamantanol, in which high specific area (ca. 140 m2 g−1) and channel nanoporosity derive from H‐bonding.
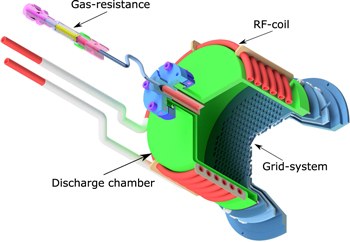
Molecular propellants for ion thrusters. Functionalized Nanodiamonds, part 77. Patrick Dietz, Waldemar Gärtner, Quirin Koch, Peter E. Köhler, Yan Teng, Peter J. Klar, Peter R. Schreiner, Kristof Holste
Plasma Sources Sci. Technol. 2019, 28, 084001. DOI: 10.1088/1361-6595/ab2c6cThere is no ideal atomic propellant for ion thrusters. Xenon commonly used as propellant becomes resource-critical in light of electric propulsion commercialization. Combining these considerations leads to seeking alternatives to xenon as propellant. In this review, we summarize the current literature on molecular propellants. We define two classes of molecules, group I and II, comprising
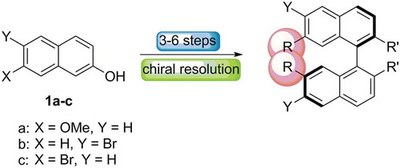
Highly efficient chirality inducers in nematic liquid crystals: synthesis of 7,7’-disubstituted 2,2’-methylenedioxy-1,1’-binaphthyls. Functionalized Nanodiamonds, part 72. Christian Kühn, Matthias Bremer and Peter R. Schreiner
Liquid Cryst. 2019, 1763-1768. DOI: 10.1080/02678292.2019.1599455
We report the synthesis of 7,7ʹ-disubstituted 2,2ʹ-methylenedioxy-1,1ʹ-binaphthyls and demonstrate their application as chiral dopants in the nematic mixture MLC-6260 to produce highly twisted cholesteric phases. Especially mesogenic and/or polarizable groups in the 7,7ʹ-positions of the bridged binaphthyls generate unusually high helical twisting powers.
Diamondoid Amino Acid-based Peptide Kinase A Inhibitor Analogues. Functionalized Nanodiamonds, part 78. Janis Müller, Romina A. Kirschner, Jan-Philipp Berndt, Tobias Wulsdorf, Alexander Metz, Radim Hrdina, Peter R. Schreiner, Armin Geyer and Gerhard Klebe
ChemMedChem 2019, 14, 663–672. DOI: 10.1002/cmdc.201800779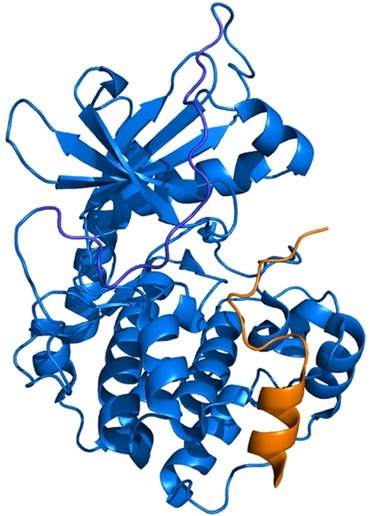
The incorporation of diamondoid amino acids (DAAs) into peptide‐like drugs is a general strategy to improve lipophilicity, membrane permeability, and metabolic stability of peptidomimetic pharmaceuticals. We designed and synthesized five novel peptidic DAA‐containing kinase inhibitors of protein kinase A using a sophisticated molecular dynamics protocol and solid‐phase peptide synthesis. By means of a thermophoresis binding assay, NMR, and crystal structure analysis, we determined the influence of the DAAs on the secondary structure and binding affinity in comparison to the native protein kinase inhibitor, which is purely composed of proteinogenic amino acids. Affinity and binding pose are largely conserved. One variant showed 6.5‐fold potency improvement, most likely related to its increased side chain lipophilicity. A second variant exhibited slightly decreased affinity presumably due to loss of hydrogen‐bond contacts to surrounding water molecules of the first solvation shell.
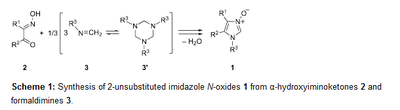
Synthesis and selected transformations of 2-unsubstituted 1-(adamantyloxy)imidazole 3-oxides: straightforward access to non-symmetric 1,3-dialkoxyimidazolium salts. Functionalized Nanodiamonds, part 77. Grzegorz Mlostoń, Małgorzata Celeda, Katarzyna Urbaniak, Vladyslav Bakhonskyi, Peter R. Schreiner and Heinz Heimgartner
Beilstein J. Org. Chem. 2019, 15, 497–505. DOI: 10.3762/bjoc.15.43Adamantyloxyamine reacts with formaldehyde to give N-(adamantyloxy)formaldimine as a room-temperature-stable compound that exists in solution in monomeric form. This product was used for reactions with α-hydroxyiminoketones leading to a new class of 2-unsubstituted imidazole 3-oxides bearing the adamantyloxy substituent at N(1). Their reactions with 2,2,4,4-tetramethylcyclobutane-1,3-dithione or with acetic acid anhydride occurred analogously to those of 1-alkylimidazole 3-oxides to give imidazol-2-thiones and imidazol-2-ones, respectively. Treatment of 1-(adamantyloxy)imidazole 3-oxides with Raney-Ni afforded the corresponding imidazole derivatives without cleavage of the N(1)–O bond. Finally, the O-alkylation reactions of the new imidazole N-oxides with 1-bromopentane or 1-bromododecane open access to diversely substituted, non-symmetric 1,3-dialkoxyimidazolium salts. Adamantyloxyamine reacts with glyoxal and formaldehyde in the presence of hydrobromic acid yielding symmetric 1,3-di(adamantyloxy)-1H-imidazolium bromide in good yield. Deprotonation of the latter with triethylamine in the presence of elemental sulfur allows the in situ generation of the corresponding imidazol-2-ylidene, which traps elemental sulfur yielding a 1,3-dihydro-2H-imidazole-2-thione as the final product.
Diamantane suspended single copper atoms. Functionalized Nanodiamonds, part 75. Hong-Ying Gao, Marina Šekutor, Lacheng Liu, Alexander Timmer, Hannah Schreyer, Harry Mönig, Saeed Amirjalayer, Nataliya A. Fokina, Armido Studer, Peter R. Schreiner and Harald Fuchs
J. Am. Chem. Soc. 2019, 141, 315–322. DOI: 10.1021/jacs.8b10067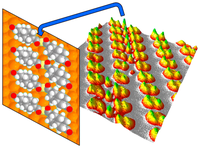
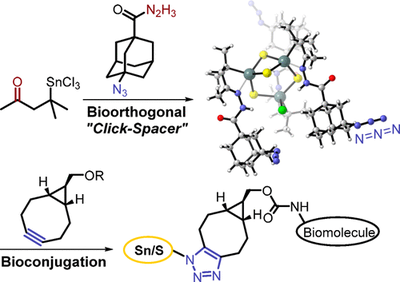
Azido-Adamantyl Tin Sulfide Clusters for Bioconjugation. Functionalized Nanodiamonds, part 73. Jan-Philipp Berndt, Annikka Engel, Radim Hrdina, Stefanie Dehnen and Peter R. Schreiner
Organometallics 2019, 38, 329–335. DOI: 10.1021/acs.organomet.8b00734We present a new versatile route toward biomolecule-functionalized tin sulfide clusters. A novel bifunctional orthogonal spacer was developed and used for the formation of a trifold azido-adamantyl-terminated cluster, serving as a building block for click reactions. The azido cluster was quantitatively bioconjugated via a strain-promoted 1,3-dipolar cycloaddition, affording a peptide-decorated cluster.
Aerobic Aliphatic Hydroxylation Reactions by Copper Complexes: A Simple Clip-and-Cleave Concept. Jonathan Becker, Yevheniia Y. Zhyhadlo, Ekaterina D. Butova, Andrey A. Fokin, Fabian M. Metz, Peter R. Schreiner, Moritz Förster, Max C. Holthausen, Pascal Specht, and Siegfried Schindler
Chem. Eur. J. 2018, 24, 15543–15549. DOI: 10.1002/chem.201802607
Highlights: a) Front cover of this issue; b) Featured in Chemviews October 09, 2018.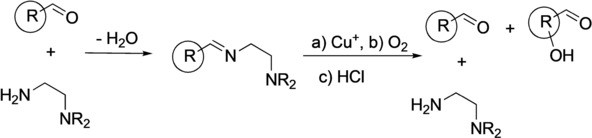
A simple imine clip‐and‐cleave concept has been developed for the selective hydroxylation of non‐activated CH bonds in aliphatic aldehydes with dioxygen through a copper complex. The synthetic protocol involves reaction of a substrate aldehyde with N,N‐diethyl‐ethylendiamine to give the corresponding imine, which is used as a bidentate donor ligand forming a copper(I) complex with [Cu(CH3CN)4][CF3SO3]. After exposure of the reaction mixture to dioxygen acidic cleavage and aqueous workup liberates the corresponding β‐hydroxylated aldehyde. The concept for the hydroxylation of trimethylacetaldehyde as well as adamantane and diamantane 1‐carbaldehydes was investigated and the corresponding β‐hydroxy aldehydes were obtained with high selectivities. The results of low temperature stopped‐flow measurements indicate the formation of a bis(μ‐oxido)dicopper complex as reactive intermediate. According to density functional theory (DFT, RI‐BLYP‐D3/def2‐TZVP(SDD)/ COSMO(CH2Cl2)//RI‐PBE‐D3/def2‐TZVP(SDD)) computations CH bonds suitably predisposed to the [Cu2O2]2+ core undergo hydroxylation in a concerted step with particularly low activation barriers, which explains the experimentally observed regioselectivities.
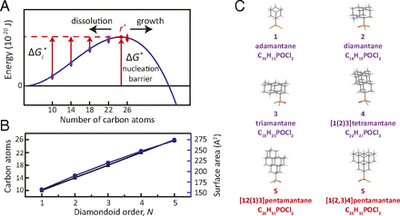
Experimental measurement of the diamond nucleation landscape reveals classical and nonclassical features. Functionalized Nanodiamonds, part 67. Matthew A. Gebbie, Patrick McQuade, Hitoshi Ishiwata, Vaclav Petrak, Andy Taylor, Jeremy E. Dahl, Robert M. K. Carlson, Andrey A. Fokin, Peter R. Schreiner, Zhi-Xun Shen, Milos Nesladek, and Nicholas A. Melosh
PNAS 2018, 115, 8284–8289. DOI: 10.1073/pnas.1803654115Nucleation is a core scientific concept that describes the formation of new phases and materials. While classical nucleation theory is applied across wide-ranging fields, nucleation energy landscapes have never been directly measured at the atomic level, and experiments suggest that nucleation rates often greatly exceed the predictions of classical nucleation theory. Multistep nucleation via metastable states could explain unexpectedly rapid nucleation in many contexts, yet experimental energy landscapes supporting such mechanisms are scarce, particularly at nanoscale dimensions. In this work, we measured the nucleation energy landscape of diamond during chemical vapor deposition, using a series of diamondoid molecules as atomically defined protonuclei. We find that 26-carbon atom clusters, which do not contain a single bulk atom, are postcritical nuclei and measure the nucleation barrier to be more than four orders of magnitude smaller than prior bulk estimations. These data support both classical and nonclassical concepts for multistep nucleation and growth during the gas-phase synthesis of diamond and other semiconductors. More broadly, these measurements provide experimental evidence that agrees with recent conceptual proposals of multistep nucleation pathways with metastable molecular precursors in diverse processes, ranging from cloud formation to protein crystallization, and nanoparticle synthesis.
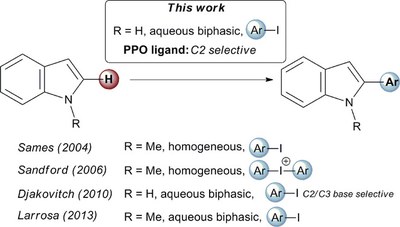
Palladium catalysed C2–H arylation of unprotected (N–H)-indoles in water using primary diamantyl phosphine oxides as a new class of PPO ligands. Functionalized Nanodiamonds, part 71. Oana Moncea, Didier Poinsot, Andrey A. Fokin, Peter. R. Schreinerand Jean-Cyrille Hierso
ChemCatChem 2018, 10, 2915-2922. DOI: 10.1002/cctc.201800187We present the palladium‐catalyzed arylation of (N‐H)‐indoles with functionalized haloarenes "on water" using hitherto untested primary diamantyl phosphine oxides (PPO) as ligands. Remarkable C2‐H arylation selectivity was achieved employing functionalized iodoarenes and N‐unprotected indoles. We provide evidence that the in situ generated oxide derivative of (9‐hydroxydiamant‐4‐yl)phosphine L1 is key for the reaction efficiency by comparison of a set of diamantane‐based compounds structurally related to L1. Our results demonstrate the power of the new PPO ligands for the C‐H functionalization of unprotected (N‐H)‐heterocycles.
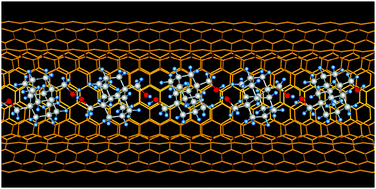
One-dimensional hydrogen bonding network of bis-hydroxylated diamantane formed inside double-wall carbon nanotubes. Functionalized Nanodiamonds, part 70. Y. Nakanishi, H. Omachi, N. A. Fokina, P. R. Schreiner, J. Becker, J. E. P. Dahl, R. M. K. Carlson and H. Shinohara
Chem. Commun. 2018, 54, 3823-3826. DOI: 10.1039/c7cc09832d
Highlight: Back cover of corresponding issue.1,6-Bis(hydroxymethyl)diamantane spontaneously aligns inside double-walled carbon nanotubes. The encapsulated molecules form a one-dimensional network within the double-walled carbon nanotubes through hydrogen bonding that leads to a highly dense filling as compared to unfunctionalized diamantane. Improving the encapsulation yields of precursors via functionalization is crucial to prepare novel one-dimensional materials.
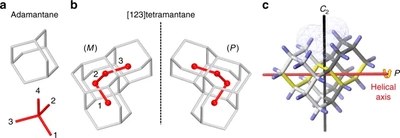
Assigning the absolute configuration of single aliphatic molecules by visual inspection. Functionalized Nanodiamonds, part 69. Daniel Ebeling, Marina Šekutor, Marvin Stiefermann, Jalmar Tschakert, Jeremy E. P. Dahl, Robert M. K. Carlson, André Schirmeisen and Peter R. Schreiner
Nat. Commun. 2018, 9, 2420. DOI: 10.1038/s41467-018-04843-zDeciphering absolute configuration of a single molecule by direct visual inspection is the next step in compound identification, with far-reaching implications for medicinal chemistry, pharmacology, and natural product synthesis. We demonstrate the feasibility of this approach utilizing low temperature atomic force microscopy (AFM) with a CO-functionalized tip to determine the absolute configuration and orientation of a single, adsorbed [123]tetramantane molecule, the smallest chiral diamondoid. We differentiate between single enantiomers on Cu(111) by direct visual inspection, and furthermore identify molecular dimers and molecular clusters. The experimental results are confirmed by a computational study that allowed quantification of the corresponding intermolecular interactions. The unique toolset of absolute configuration determination combined with AFM tip manipulation opens a route for studying molecular nucleation, including chirality-driven assembly or reaction mechanisms.
White-light generation through nonlinear optical response of 1,3,5,7-tetraphenyladamantane: Amorphous vs. crystalline states. Functionalized Nanodiamonds, part 68. Nils W. Rosemann, Harald Locke, Peter R. Schreiner and Sangam Chatterjee
Adv. Opt. Mat. 2018, 1701162. DOI: 10.1002/adom.201701162The vast optical nonlinearity of 1,3,5,7‐tetraphenyladamantane (AdPh4) enables efficient frequency conversion. The observed spectrum depends heavily on the material's habitus. In particular, it shows nonlinear white‐light generation when its powder form is irradiated in the near‐infrared, similar to an analogous organotin sulfide cluster [(PhSn)4S6]. In contrast, single crystals of AdPh4 exclusively exhibit narrow‐band second‐harmonic generation, i.e., discrete conversion within the crystal, rather than spectrally broadband white‐light emission. This shows that crystallinity restricts the nonlinear response to the symmetry‐allowed higher‐harmonics, in this case the even‐numbered, second harmonic, and inhibits the quasi‐continuum white‐light emission. Using the diamondoid‐based material significantly enhances compatibility with conventional production techniques using photorefractive polymers and is thus an important step towards the fabrication of all‐integrated white‐light emitting devices.
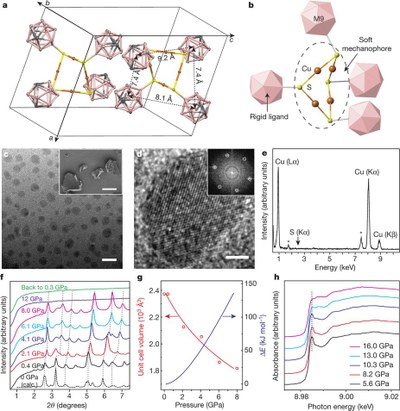
Sterically-controlled mechanochemistry under hydrostatic pressure. Functionalized Nanodiamonds, part 66. Hao Yan, Fan Yang, Ding Pan, Yu Lin, J. Nathan Hohman, Diego Solis-Ibarra, Fei Hua Li, Jeremy E. P. Dahl, Robert M. K. Carlson, Boryslav A. Tkachenko, Andrey A. Fokin, Peter R. Schreiner, Giulia Galli, Wendy L. Mao, Zhi-Xun Shen and Nicholas A. Melosh
Nature 2018, 554, 505-510. DOI: 10.1038/nature25765
Highlights: a) “Pressure squeezes reduction reactions out of crystals.” Chem. Eng. News 2018, 96(9), p. 6 (“News of the Week); b) “In a first, tiny diamond anvils trigger chemical reactions by squeezing” phys.org, Feb 21, 2018.Mechanical stimuli can modify the energy landscape of chemical reactions and enable reaction pathways, offering a synthetic strategy that complements conventional chemistry1,2,3. These mechanochemical mechanisms have been studied extensively in one-dimensional polymers under tensile stress4,5,6,7,8,9 using ring-opening10 and reorganization11, polymer unzipping6,12 and
Monochromatic photocathodes from graphene-stabilized diamondoids. Functionalized Nanodiamonds, part 65. Hao Yan, Karthik T. Narashimha, Jonathan Denlinger, Fei Hua Li, Sung-Kwan Mo, J. Nathan Hohman, Jeremy E. P. Dahl, Robert M. K. Carlson, Boryslav A. Tkachenko, Andrey A. Fokin, Peter R. Schreiner, Zahid Hussain, Zhi-Xun Shen and Nicholas A. Melosh
Nano Lett. 2018, 18, 1099-1103. DOI: 10.1021/acs.nanolett.7b04645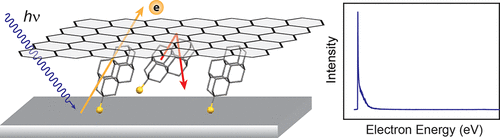
The monochromatic photoemission from diamondoid monolayers provides a new strategy to create electron sources with low energy dispersion and enables compact electron guns with high brightness and low beam emittance for aberration-free imaging, lithography, and accelerators. However, these potential applications are hindered by degradation of diamondoid monolayers under photon irradiation and electron bombardment. Here, we report a graphene-protected diamondoid monolayer photocathode with 4-fold enhancement of stability compared to the bare diamondoid counterpart. The single-layer graphene overcoating preserves the monochromaticity of the photoelectrons, showing 12.5 meV ful width at half-maximum distribution of kinetic energy. Importantly, the graphene coating effectively suppresses desorption of the diamondoid monolayer, enhancing its thermal stability by at least 100 K. Furthermore, by comparing the decay rate at different photon energies, we identify electron bombardment as the principle decay pathway for diamondoids under graphene protection. This provides a generic approach for stabilizing volatile species on photocathode surfaces, which could greatly improve performance of electron emitters.
Nanodiamond‐Palladium Core–Shell Organohybrid Synthesis: A Mild Vapor‐Phase Procedure Enabling Nanolayering Metal onto Functionalized sp3‐Carbon. Functionalized Nanodiamonds, part 64. Maria A. Gunawan, Oana Moncea, Didier Poinsot, Mariem Keskes, Bruno Domenichini, Olivier Heintz, Rémi Chassagnon, Frédéric Herbst, Jeremy E. P. Dahl, Andrey A. Fokin, Peter R. Schreiner and Jean-Cyrille Hierso
Adv. Funct. Mat. 2018, 1705786/1–15. DOI: 10.1002/adfm.201705786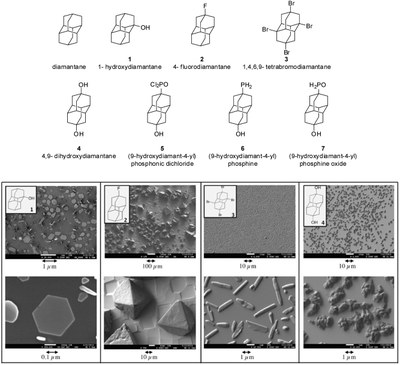
Electronic and Vibrational Properties of Diamondoid Oligomers. Functionalized Nanodiamonds, part 60. Christoph Tyborski, Roland Gillen, Andrey A. Fokin, Natalie A. Fokina, Tetyana V. Koso, Heike Hausmann, Vladimir N. Rodionov, Peter R. Schreiner, Christian Thomsen and Janina Maultzsch
J. Chem. Phys. C 2017, 121, 27082–27088. DOI: 10.1021/acs.jpcc.7b07666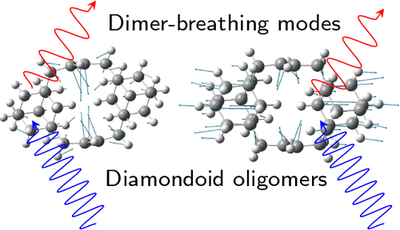
We analyzed the vibrational and electronic properties of diamondoid oligomers via resonance Raman spectroscopy. The compounds consist of lower diamondoids such as adamantane or diamantane that are interconnected with double bonds. Therefore, all oligomers have ethylene-like centers strongly influencing the character of the optical transitions. The double bond localizes the HOMO (highest occupied moluecular orbital) in between the diamondoids accompanied by a significant decrease of optical transition energies. Comparing Raman spectra of the compounds to pristine diamondoids, we find several characteristic modes originating from the ethylene moieties. Supported by DFT (density functional theory) computations, we attribute these modes to highly localized vibrations that can partially be derived from the vibrational modes of parent ethylene. We further observe two new Raman modes in the compounds: a dimer breathing mode and a rotational mode of the entire ethylene moieties.
Intramolecular London Dispersion Interaction Effects on Gas Phase and Solid State Structures of Diamondoid Dimers. Functionalized Nanodiamonds, part 61. Andrey A. Fokin, Tatyana S. Zhuk, Sebastian Blomeyer, Cristobal Perez, Lesya V. Chernish, Alexander E. Pashenko, Jens Antony, Yury V. Vishnevskiy, Raphael J. F. Berger, Stefan Grimme, Christian Logemann, Melanie Schnell, Norbert W. Mitzel and Peter R. Schreiner
J. Am. Chem. Soc. 2017, 139, 16696–16707. DOI: 10.1021/jacs.7b07884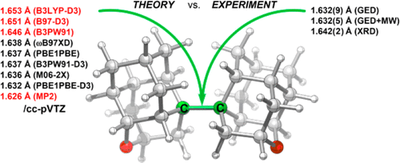
The covalent diamantyl (C28H38) and oxadiamantyl (C26H34O2) dimers are stabilized by London dispersion attractions between the dimer moieties. Their solid-state and gas-phase structures were studied using a multitechnique approach, including single-crystal X-ray diffraction (XRD), gas-phase electron diffraction (GED), a combined GED/microwave (MW) spectroscopy study, and quantum chemical calculations. The inclusion of medium-range electron correlation as well as the London dispersion energy in density functional theory is essential to reproduce the experimental geometries. The conformational dynamics computed for C26H34O2 agree well with solution NMR data and help in the assignment of the gas-phase MW data to individual diastereomers. Both in the solid state and the gas phase the central C–C bond is of similar length for the diamantyl [XRD, 1.642(2) Å; GED, 1.630(5) Å] and the oxadiamantyl dimers [XRD, 1.643(1) Å; GED, 1.632(9) Å; GED+MW, 1.632(5) Å], despite the presence of two oxygen atoms. Out of a larger series of quantum chemical computations, the best match with the experimental reference data is achieved with the PBEh-3c, PBE0-D3, PBE0, B3PW91-D3, and M06-2X approaches. This is the first gas-phase confirmation that the markedly elongated C–C bond is an intrinsic feature of the molecule and that crystal packing effects have only a minor influence.
Host-guest complexes of cyclodextrins and nanodiamonds as strong non-covalent binding motif for self-assembled nanomaterials. Functionalized Nanodiamonds, part 63. Frauke Schibilla, Jens Voskuhl, Natalie A. Fokina, Jeremy E. P. Dahl, Peter R. Schreiner and Bart Jan Ravoo
Chem. Eur. J. 2017, 23, 16059–16065. DOI: 10.1002/chem.201703392
London dispersion directs on-surface self-assembly of [121]tetramantane molecules. Daniel Ebeling, Marina Šekutor, Marvin Stiefermann, Jalmar Tschakert, Jeremy E. P. Dahl, Robert M. K. Carlson, André Schirmeisen and Peter R. Schreiner
ACS Nano 2017, 11, 9459–9466. DOI: 10.1021/acsnano.7b05204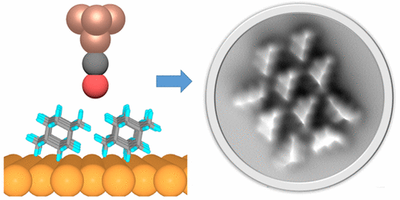
London dispersion (LD) acts between all atoms and molecules in nature, but the role of LD interactions in the self-assembly of molecular layers is still poorly understood. In this study, direct visualization of single molecules using atomic force microscopy with CO-functionalized tips revealed the exact adsorption structures of bulky and highly polarizable [121]tetramantane molecules on Au(111) and Cu(111) surfaces. We determined the absolute molecular orientations of the completely sp3-hybridized tetramantanes on metal surfaces. Moreover, we demonstrate how LD drives this on-surface self-assembly of [121]tetramantane hydrocarbons, resulting in the formation of a highly ordered 2D lattice. Our experimental findings were underpinned by a systematic computational study, which allowed us to quantify the energies associated with LD interactions and to analyze intermolecular close contacts and attractions in detail.
From isolated molecules to a van-der-Waals crystal: A theoretical and experimental analysis of a trishomocubane and a diamantane dimer in the gas and in the solid phase. Functionalized Nanodiamonds, part 59. Christoph Tyborski, Reinhard Meinke, Roland Gillen, Tobias Zimmermann, Andre Knecht, Torbjörn Rander, Robert Richter, Andrea Merli, Andrey A. Fokin, Tetyana V. Koso, Vladimir N. Rodionov, Peter R. Schreiner, Thomas Möller, Christian Thomsen, and Janina Maultzsch J. Chem. Phys. 2017, 147, 044303. DOI: 10.1063/1.4994898
The electronic properties of sp2/sp3 diamondoids in the crystalline state and in the gas phase are presented. Apparent differences in electronic properties experimentally observed by resonance Raman spectroscopy in the crystalline/gas phase and absorption measurements in the gas phase

Chiral Building Blocks Based on 1,2-Disubstituted Diamantane. Functionalized Nanodiamonds, part 58. Andrey A. Fokin,* Alexander E. Pashenko, Vladyslav V. Bakhonsky, Tatyana S. Zhuk, Lesya V. Chernish, Pavel A. Gunchenko, Andrey O. Kushko, Jonathan Becker, Raffael C. Wende and Peter R. Schreiner*
Synthesis 2017, 49, 2003–2008. DOI: 10.1055/s-0036-1588694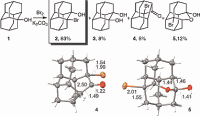
Vertical-Substrate MPCVD Epitaxial Nano-diamond Growth. Functionalized Nanodiamonds, part 57. Yan-Kai Tzeng, Jingyuan Linda Zhang, Haiyu Lu, Hitoshi Ishiwata, Jeremy E. P. Dahl, Robert M. K. Carlson, Hao Yan, Peter R. Schreiner, Jelena Vuckovic, Zhi-Xun Shen, Nicholas A. Melosh, Steven Chu
Nano Lett. 2017, 17, 1489–1495. DOI: 10.1021/acs.nanolett.6b04543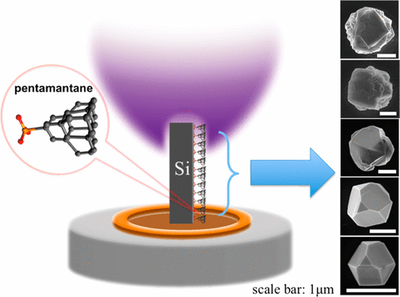
Color center-containing nanodiamonds have many applications in quantum technologies and biology. Diamondoids, molecular-sized diamonds have been used as seeds in chemical vapor deposition (CVD) growth. However, optimizing growth conditions to produce high crystal quality nanodiamonds with color centers requires varying growth conditions that often leads to ad-hoc and time-consuming, one-at-a-time testing of reaction conditions. In order to rapidly explore parameter space, we developed a microwave plasma CVD technique using a vertical, rather than horizontally oriented stage-substrate geometry. With this configuration, temperature, plasma density, and atomic hydrogen density vary continuously along the vertical axis of the substrate. This variation allowed rapid identification of growth parameters that yield single crystal diamonds down to 10 nm in size and 75 nm diameter optically active center silicon-vacancy (Si-V) nanoparticles. Furthermore, this method may provide a means of incorporating a wide variety of dopants in nanodiamonds without ion irradiation damage.
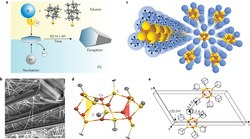
Hybrid metal–organic chalcogenide nanowires with electrically conductive inorganic core through diamondoid-directed assembly. Functionalized Nanodiamonds, Part 54. Hao Yan, J. Nathan Hohman, Fei Hua Li, Chunjing Jia, Diego Solis-Ibarra, Jeremy E. P. Dahl, Robert M. K. Carlson, Boryslav A. Tkachenko, Andrey A. Fokin, Peter R. Schreiner,* Yufeng Liang, Taheo Roy Kim, Thomas Devereaux, Zhi-Xun Shen,* and Nicholas A. Melosh,*
Nature Mat. 2017, 16, 349–357. DOI: 10.1038/nmat4823Controlling inorganic structure and dimensionality through structure-directing agents is a versatile approach for new materials synthesis that has been used extensively for metal–organic frameworks and coordination polymers. However, the lack of ‘solid’ inorganic cores requires charge
Defying Stereotypes with Nanodiamonds: Stable Primary Diamondoid Phosphines. Functionalized Nanodiamonds, part 56. Oana Moncea, Maria A. Gunawan, Didier Poinsot, Hélène Cattey, Jonathan Becker, Raisa I. Yurchenko, Ekaterina D. Butova, Heike Hausmann, Marina Šekutor, Andrey A. Fokin,* Jean-Cyrille Hierso,* and Peter R. Schreiner*
J. Org. Chem. 2016, 81, 8759–8769. DOI: 10.1021/acs.joc.6b01219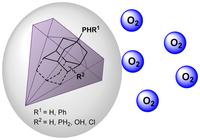
Peptide-Functionalized Organotin Sulfide Clusters. Niklas Rinn, Jan-Philipp Berndt, Annikka Kreher, Natalie Brüll, Radim Hrdina, Matthias Reinmuth, Peter R. Schreiner and Stefanie Dehnen*
Organometallics 2016, 35, 3215–3220. DOI: 10.1021/acs.organomet.6b00561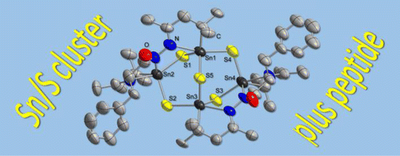
We report the first successful attachment of peptides to tin sulfide clusters. For proof of principle, H-l-Phe hydrazide and Boc-protected dipeptide hydrazides (Boc-l-Ala-l-Ala hydrazide and Boc-l-Val-l-Phe hydrazide) were reacted with keto-functionalized tin sulfide clusters [(R1Sn)4S6] (A; R1 = CMe2CH2C(O)Me) and [(R1Sn)3S4Cl] (B). In the first case, we obtained single crystals of an amino acid functionalized Sn/S cluster, [R22Sn4S5] (1; R2 = (CMe2CH2C(Me)N2C(O)CH(CH2Ph)NC(Me)CH2CMe2), formed after inorganic cluster rearrangement and intramolecular condensation of the amino acid ligand. By means of NMR spectroscopic investigations and ESI/LIFDI mass spectrometry, we demonstrate that both dipeptides are attached to B under retention of the original cluster architecture to yield [(R3Sn)3S4Cl] (2; R3 = CMe2CH2C(NNH-Ala-Ala-Boc)Me) and [(R4Sn)3S4Cl] (3; R4 = CMe2CH2C(NNH-Phe-Val-Boc)Me), as evident from mass spectrometric data of their cations [(R3Sn)3S4]+ (2+) and [(R4Sn)3S4]+ (3+).
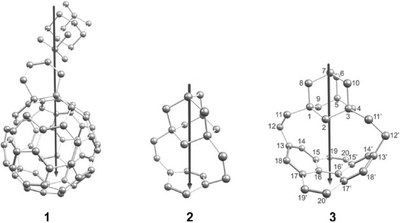
[2](1,3)Adamantano[2]-(2,7)pyrenophane, a Hydrocarbon with a Large Dipole Moment. Functionalized Nanodiamonds, part 55. Paul Kahl, Jan P. Wagner, Ciro Balestrieri, Jonathan Becker, Heike Hausmann, Graham J. Bodwell and Peter R. Schreiner
Angew. Chem. Int. Ed. 2016, 55, 9277-9281. DOI: 10.1002/anie.201602201The fusion of the sp3-hybridized parent diamondoid adamantane with the sp2-hybridized pyrene results in a hybrid structure with a very large dipole moment which arises from bending the pyrene moiety. Presented herein is the synthesis, study of the electronic and optical properties, as well as the dynamic behavior of this new hydrocarbon.
Hybrid group IV nanophotonic structures incorporating diamond silicon-vacancy color centers. Functionalized Nanodiamonds, part 52.
Jingyuan Linda Zhang, Hitoshi Ishiwata, Thomas M. Babinec, Marina Radulaski, Kai Müller, Konstantinos G. Lagoudakis, Jeremy E. P. Dahl, Robert Edgington, Veronique Souliére, Gabriel Ferro, Andrey A. Fokin, Peter R. Schreiner, Zhi-Xun Shen, Nick Melosh and Jelena Vukovic
Nano Lett. 2016, 15, 212–217. DOI: 10.1021/acs.nanolett.5b03515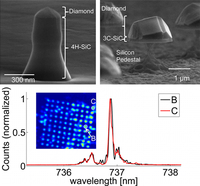
Ultralow effective work function surfaces using diamondoid monolayers. Functionalized Nanodiamonds, part 51.
Karthik T. Narasimha, Chenhao Ge, Jason D. Fabbri, William Clay, Boryslav A. Tkachenko, Andrey A. Fokin, Peter R. Schreiner, Jeremy E. Dahl, Robert M. K. Carlson, Z. X. Shen, Nicholas A. Melosh
Nature Nanotech. 2016, 11, 267–272. DOI: 10.1038/nnano.2015.277Electron emission is critical for a host of modern fabrication and analysis applications including mass
 spectrometry, electron imaging and nanopatterning. Here, we report that monolayers of diamondoids effectively confer dramatically enhanced field emission properties to metal surfaces. We attribute the improved emission to a significant reduction of the work function rather than a geometric enhancement. This effect depends on the particular diamondoid isomer, with [121]tetramantane-2-thiol reducing gold's work function from ∼5.1 eV to 1.60 ± 0.3 eV, corresponding to an increase in current by a factor of over 13,000. This reduction in work function is the largest reported for any organic species and also the largest for any air-stable compound1, 2, 3. This effect was not observed for sp3-hybridized alkanes, nor for smaller diamondoid molecules. The magnitude of the enhancement, molecule specificity and elimination of gold metal rearrangement precludes geometric factors as the dominant contribution. Instead, we attribute this effect to the stable radical cation of diamondoids. Our computed enhancement due to a positively charged radical cation was in agreement with the measured work functions to within ±0.3 eV, suggesting a new paradigm for low-work-function coatings based on the design of nanoparticles with stable radical cations.
spectrometry, electron imaging and nanopatterning. Here, we report that monolayers of diamondoids effectively confer dramatically enhanced field emission properties to metal surfaces. We attribute the improved emission to a significant reduction of the work function rather than a geometric enhancement. This effect depends on the particular diamondoid isomer, with [121]tetramantane-2-thiol reducing gold's work function from ∼5.1 eV to 1.60 ± 0.3 eV, corresponding to an increase in current by a factor of over 13,000. This reduction in work function is the largest reported for any organic species and also the largest for any air-stable compound1, 2, 3. This effect was not observed for sp3-hybridized alkanes, nor for smaller diamondoid molecules. The magnitude of the enhancement, molecule specificity and elimination of gold metal rearrangement precludes geometric factors as the dominant contribution. Instead, we attribute this effect to the stable radical cation of diamondoids. Our computed enhancement due to a positively charged radical cation was in agreement with the measured work functions to within ±0.3 eV, suggesting a new paradigm for low-work-function coatings based on the design of nanoparticles with stable radical cations.Template Synthesis of Linear Chain Nanodiamonds Inside Carbon Nanotubes from Bridgehead-Halogenated Diamantane Precursors. Funtionalized Nanodiamonds, part 50.
Yusuke Nakanishi, Haruka Omachi, Natalie A. Fokina, Ryo Kitaura, Peter R. Schreiner, Jeremy E. P. Dahl, Robert M. K. Carlson, and Hisanori Shinohara
Angew. Chem. Int. Ed. 2015, 54, 10802–10806. DOI: 10.1002/anie.201504904
Highlights: a) Noted as hot paper (top 10% of all Angewandte publications); b) Nanodiamonds lined up in ChemistryViews, September 02, 2015; c) Auffädelung von Nanodiamanten auf chemie.de, September 03, 2015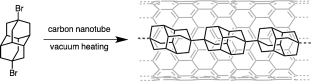
A simple method for the synthesis of linear-chain diamond-like nanomaterials, so-called diamantane polymers, is described. This synthetic approach is primarily based on a template reaction of dihalogen-substituted diamantane precursors in the hollow cavities of carbon nanotubes. Under high vacuum and in the presence of Fe nanocatalyst particles, the dehalogenated radical intermediates spontaneously form linear polymer chains within the carbon nanotubes. Transmission electron microscopy reveals the formation of well-aligned linear polymers. We expect that the present template-based approach will enable the synthesis of a diverse range of linear-chain polymers by choosing various precursor molecules. The present technique may offer a new strategy for the design and synthesis of one-dimensional nanomaterials.
Transition metal complexes with cage-opened diamondoidtetracyclo[7.3.1.14,14.02,7]tetra-deca-6,11-diene. Functionalized Nanodiamonds, part 49.
Lars Valentin, Anja Henss, Boryslav A. Tkachenko, Andrey A. Fokin, Peter R. Schreiner, Sabine Becker, Christian Würtele, and Siegfried Schindler
J. Coord. Chem. 2015, 68, 3295–3301. DOI: 10.1080/00958972.2015.1071802Cage-opened diamondoid tetracyclo[7.3.1.14,12.02,7]tetradeca-6,11-diene forms complexes with AgNO3 and CuCl. The latter crystallized from acetonitrile in polymeric form [Cu2Cl2(CH3CN)(diene)]n; in the presence of 2,2′-bipyridine, a double-charged monomeric Cu(I)-complex [Cu2(bipy)2(diene)]2+ formed. Both complexes were structurally characterized through X-ray crystal diffraction analysis.Copper(I) complexes with the diamantane diene tetracyclo[7.3.1.14,12.02,7]tetradeca-6.11-diene (6) have been prepared and an interesting crystal structure, [Cu2(bipy)2(6)]2+ (hydrogen atoms, anions, and solvent molecules are omitted for clarity), was obtained.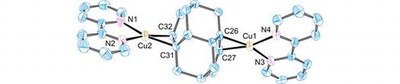
Inverted Carbon Geometries: Challenges to Experiment and Theory.
Matthias Bremer, Harald Untenecker, Pavel A. Gunchenko, Andrey A. Fokin, and Peter R. Schreiner
J. Org. Chem. 2015, 80, 6520–6524. DOI: 10.1021/acs.joc.5b00845
Highlights: a) Clare Tovee, Cambridge Structural Database (CCDC), June 18, 2015; b) Steven Bachrach, Computational Organic Chemistry, July 6, 2016.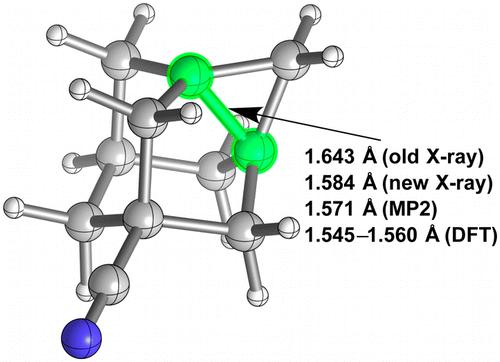
Disproving a long C–C-bond textbook example: The reported 1.643 Å C–C bond in 5-cyano-1,3-dehydroadamantane was redetermined and “only” amounts to 1.584 Å. While this value is well reproduced with ab initio methods, some common DFT approaches perform poorly and are only consistent with CCSD(T)/cc-pVTZ optimizations for noninverted carbons. Large deviations from experiment were also found for other molecules with atypical electron density distributions, e.g., cubane, bicyclo[2.2.0]hexane, and bicyclo[2.1.0]- and bicyclo[1.1.1]pentane, thereby presenting challenging structures for some DFT implementations.
Toward an Understanding of Diamond sp2-Defects with Unsaturated Diamondoid Oligomer Models. Tatyana S. Zhuk, Tatyana Koso, Alexander E. Pashenko, Ngo Trung Hoc, Vladimir N. Rodionov, Michael Serafin, Peter R. Schreiner and Andrey A Fokin
J. Am. Chem. Soc. 2015, 137, 6577–6586. DOI: 10.1021/jacs.5b01555
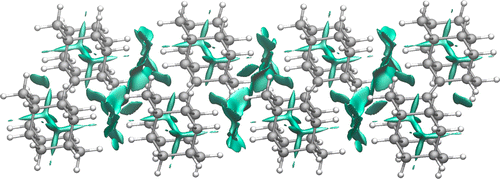
Nanometer-sized doubly bonded diamondoid dimers and trimers, which may be viewed as models of diamond with surface sp2-defects, were prepared from corresponding ketones via a McMurry coupling and were characterized by spectroscopic and crystallographic methods. The neutral hydrocarbons and their radical cations were studied utilizing density functional theory (DFT) and ab initio (MP2) methods, which reproduce the experimental geometries and ionization potentials well. The van der Waals complexes of the oligomers with their radical cations that are models for the self-assembly of diamondoids, form highly delocalized and symmetric electron-deficient structures. This implies a rather high degree of σ-delocalization within the hydrocarbons, not too dissimilar to delocalized π-systems. As a consequence, sp2-defects are thus also expected to be nonlocal, thereby leading to the observed high surface charge mobilities of diamond-like materials. In order to be able to use the diamondoid oligomers for subsequent surface attachment and modification, their C—H-bond functionalizations were studied, and these provided halogen and hydroxy derivatives with conservation of unsaturation.
The functionalization of nanodiamonds (diamondoids) as a key parameter of their easily controlled self-assembly in micro- and nanocrystals from the vapor phase. Functionalized Nanodiamonds, part 43. Maria A. Gunawan, Didier Poinsot, Bruno Domenichini, Sébastien Chevalier, Céline Dirand, Andrey A. Fokin, Peter R. Schreiner, and Jean-Cyrille Hierso
Nanoscale 2015, 7, 1956-1962. DOI: 10.1039/C4NR04442H. Open Access.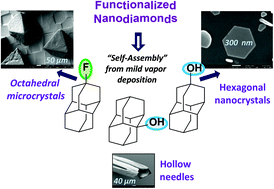
We detail herein readily accessible processes to control previously unobserved robust self-assemblies of nanodiamonds (diamondoids) in micro- and nanocrystals from their mild vapor deposition. The chemical functionalization of uniform and discernible nanodiamonds was found to be a key parameter, and depending on the type of functional group (hydroxy, fluorine, etc.) and its position on the diamondoid, the structure of the discrete deposits can vary dramatically. Thus, well-defined anisotropic structures such as rod, needle, triangle or truncated octahedron shapes can be obtained, and self-assembled edifices of sizes ranging from 20 nm to several hundred micrometers formed with conservation of a similar structure for a given diamondoid. Key thermodynamic data including sublimation enthalpy of diamondoid derivatives are reported, and the SEM of the self-assemblies coupled with EDX analyses and XRD attest the nature and purity of nanodiamond crystal deposits. This attractive method is simple and outperforms in terms of deposit quality dip-coating methods we used. This vapor phase deposition approach is expected to allow for an easy formation of diamondoid nanoobjects on different types of substrates.
Beyond the Corey Reaction II: Dimethylenation of Sterically Congested Ketones. Anastasiya V. Barabash, Ekaterina D. Butova, Igor M. Kanyuk, Peter R. Schreiner, and Andrey A. Fokin
J. Org. Chem. 2014, 79, 10669–10673. DOI: 10.1021/jo502021x
Highlight: selected as “Editor’s choice” and made open access by the journal. Listed as one of the five most accessed JOC articles in 2014.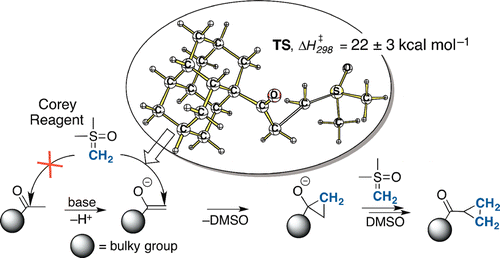
Bulky methyl ketones show significantly decreased reactivities toward the Corey-Chaykovsky methylenation reagent dimethylsulfoxonium methylide (DMSM). The excess of base and temperature increase opens an alternative reaction channel that instead leads to the corresponding cyclopropyl ketones. Computations suggest that the initial reaction step involves the methylene group transfer from DMSM on the ketone enolate followed by the intramolecular cyclization. The key step is associated with a barrier of 22 ± 3 kcal mol–1 and is driven by exothermic elimination of DMSO.
Unconventional Molecule-Resolved Current Rectification in Diamondoid-Fullerene Hybrids. Functionalized Nanodiamonds, part 44. Jason C. Randel, Francis C. Niestemski, Andrés R. Botello-Mendez, Warren Mar, Georges Ndabashimiye, Sorin Melinte, Jeremy E. P. Dahl, Robert M. K. Carlson, Ekaterina D. Butova, Andrey A. Fokin, Peter R. Schreiner, Jean-Christophe Charlier, and Hari C. ManoharanHighlights:b) Innovations Report: Wichtiger Baustein für die molekulare organische Elektronik erstmals charakterisiert.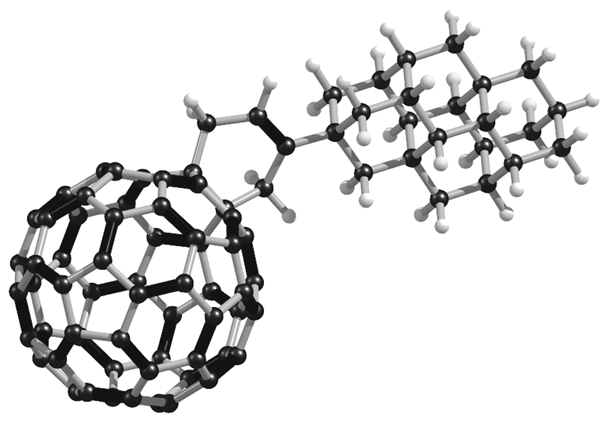 The unimolecular rectifier is a fundamental building block of molecular electronics. Rectification in single molecules can arise from electron transfer between molecular orbitals displaying asymmetric spatial charge distributions, akin to p–n junction diodes in semiconductors. Here we report a novel all-hydrocarbon molecular rectifier consisting of a diamantane–C60 conjugate. By linking both sp3 (diamondoid) and sp2 (fullerene) carbon allotropes, this hybrid molecule opposingly pairs negative and positive electron affinities. The single-molecule conductances of self-assembled domains on Au(111), probed by low-temperature scanning tunnelling microscopy and spectroscopy, reveal a large rectifying response of the molecular constructs. This specific electronic behaviour is postulated to originate from the electrostatic repulsion of diamantane–C60 molecules due to positively charged terminal hydrogen atoms on the diamondoid interacting with the top electrode (scanning tip) at various bias voltages. Density functional theory computations scrutinize the electronic and vibrational spectroscopic fingerprints of this unique molecular structure and corroborate the unconventional rectification mechanism.Selective Preparation of Diamondoid Phosphonates. Functionalized Nanodiamonds, part 44. Andrey A. Fokin, Raisa I. Yurchenko, Boryslav A. Tkachenko, Natalie A. Fokina, Maria A. Gunawan, Didier Poinsot, Jeremy E. P. Dahl, Robert M. K. Carlson, Michael Serafin, Hélène Cattey, Jean-Cyrille Hierso, and Peter R. SchreinerJ. Org. Chem. 2014, 79, 5369–5373. DOI: 10.1021/jo500793m
The unimolecular rectifier is a fundamental building block of molecular electronics. Rectification in single molecules can arise from electron transfer between molecular orbitals displaying asymmetric spatial charge distributions, akin to p–n junction diodes in semiconductors. Here we report a novel all-hydrocarbon molecular rectifier consisting of a diamantane–C60 conjugate. By linking both sp3 (diamondoid) and sp2 (fullerene) carbon allotropes, this hybrid molecule opposingly pairs negative and positive electron affinities. The single-molecule conductances of self-assembled domains on Au(111), probed by low-temperature scanning tunnelling microscopy and spectroscopy, reveal a large rectifying response of the molecular constructs. This specific electronic behaviour is postulated to originate from the electrostatic repulsion of diamantane–C60 molecules due to positively charged terminal hydrogen atoms on the diamondoid interacting with the top electrode (scanning tip) at various bias voltages. Density functional theory computations scrutinize the electronic and vibrational spectroscopic fingerprints of this unique molecular structure and corroborate the unconventional rectification mechanism.Selective Preparation of Diamondoid Phosphonates. Functionalized Nanodiamonds, part 44. Andrey A. Fokin, Raisa I. Yurchenko, Boryslav A. Tkachenko, Natalie A. Fokina, Maria A. Gunawan, Didier Poinsot, Jeremy E. P. Dahl, Robert M. K. Carlson, Michael Serafin, Hélène Cattey, Jean-Cyrille Hierso, and Peter R. SchreinerJ. Org. Chem. 2014, 79, 5369–5373. DOI: 10.1021/jo500793m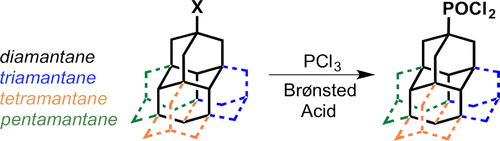 We present an effective sequence for the preparation of phosphonic acid derivatives of the diamondoids diamantane, triamantane, [121]tetramantane, and [1(2,3)4]pentamantane. The reactions of the corresponding diamondoid hydroxy derivatives with PCl3 in sulfuric or trifluoroacetic acid give mono- as well as didichlorophosphorylated diamondoids in high preparative yields.Synthesis of Substituted Adamantylzing Reagents Using a Br/Mg-Insertion in the Presence of ZnCl2 and their Subsequent Functionalization. Christoph Sämann, Vasudevan Dhayalan, Peter R. Schreiner, and Paul Knochel*
We present an effective sequence for the preparation of phosphonic acid derivatives of the diamondoids diamantane, triamantane, [121]tetramantane, and [1(2,3)4]pentamantane. The reactions of the corresponding diamondoid hydroxy derivatives with PCl3 in sulfuric or trifluoroacetic acid give mono- as well as didichlorophosphorylated diamondoids in high preparative yields.Synthesis of Substituted Adamantylzing Reagents Using a Br/Mg-Insertion in the Presence of ZnCl2 and their Subsequent Functionalization. Christoph Sämann, Vasudevan Dhayalan, Peter R. Schreiner, and Paul Knochel*Org. Lett. 2014, 136, 2418–2421. DOI: 10.1021/ol500781j.
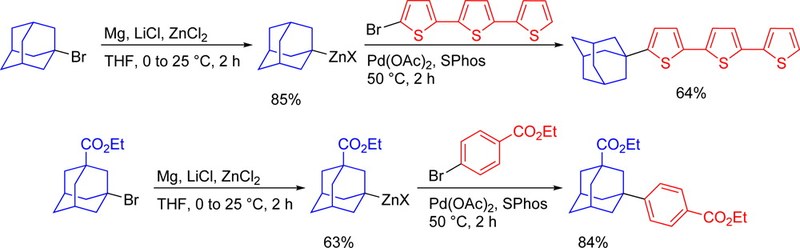
The LiCl-mediated Mg-insertion in the presence of ZnCl2 allows an efficient synthesis of adamantylzinc reagents starting from the corresponding functionalized tertiary bromides. The highly reactive adamantylzinc species readily undergo a broad variety of functionalizations such as Negishi cross-couplings, Cu(I)-catalyzed acylations and allylations, and 1,4-addition reactions leading to the expected products in excellent yields. Furthermore, the adamantyl moiety could be introduced as α-substituent in terthiophene, increasing its solubility due to the higher lipophilicity and the prevention of π-stacking.
Diamondoid hydrozones and hydrazides: sterically demanding ligands for Sn/S cluster design. Functionalized Nanodiamonds, part 42. Beatrix E. K. Barth, Boryslav A. Tkachenko, Jens P. Eußner, Peter R. Schreiner, and Stefanie Dehnen*
Organometallics 2014, 33, 1678–1688. DOI: 10.1021/om500014z.
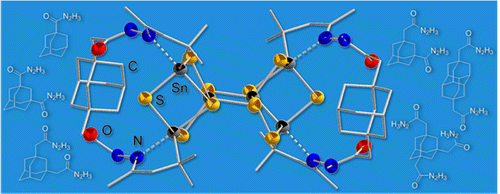
A series of new adamantane and diamantane hydrazides was synthesized and coupled with organo-functionalized Sn/S clusters of the general type [R1Sn4S6] (R1 = CMe2CH2COMe) to form diamondoid-decorated Sn/S clusters. The new ligand precursors as well as the resulting hybrid compounds were analyzed by NMR spectroscopy, mass spectrometry, and single-crystal X-ray diffraction, and first insights were gained in the installation of sterically highly demanding and at the same time rigid mono-, di-, and trifunctionalized diamondoid ligands on tetrelchalcogenide cages.
Functionalization of Homodiamantane: Oxygen Insertion Reactions Without Rearrangement with Dimethyldioxirane. Functionalized Nanodiamonds, part 42. Andrey A. Fokin,* Tanya S. Zhuk, Alexander E. Pashenko, Valeriy V. Osipov, Pavel A. Gunchenko, Michael Serafin, Peter R. Schreiner,*
J. Org. Chem. 2014, 79, 1861–1866. DOI: 10.1021/jo4026594
Homodiamantane bromination and nitroxylation are accompanied by contraction of the seven-membered ring to give the corresponding substituted 1-diamantylmethyl derivatives. In contrast, CH-bond hydroxylations with dimethyldioxirane retain the cage and give both apically and medially substituted homodiamantanes. The product ratios are in accord with the barriers for the oxygen insertion computed with density functional theory methods only if solvation is included through a polarizable continuum model. B3LYP-D3 and M06-2X computations with a 6-31G(d,p) basis set on the oligomeric van der Waals complexes predict the potential of homodiamantane derivatives for surface modifications with conformationally slightly flexible diamondoid homologues.
UV resonance Raman analysis of trishomocubane and diamondoid dimers. Functionalized Nanodiamonds, part 38. Reinhard Meinke, Robert Richter, Andrea Merli, Andrey A. Fokin, Tetyana V. Koso, Vladimir N. Rodionov, Peter R. Schreiner, Christian Thomsen, Janina Maultzsch J. Chem. Phys. 2014, 140, 034309-1–5. DOI: 10.1063/1.4861758
We present resonance Raman measurements of crystalline trishomocubane and diamantane dimers containing a C=C double bond. Raman spectra were recorded with excitation energies between 2.33 eV and 5.42 eV. The strongest enhancement is observed for the C=C stretch vibration and a bending mode involving the two carbon atoms of the C=C bond, corresponding to the B2g wagging mode of ethylene. This is associated with the localization of the π-HOMO and LUMO and the elongation of the C=C bond length and a pyramidalization of the two sp2-hybridized carbon atoms at the optical excitation. The observed Raman resonance energies of the trishomocubane and diamantane dimers are significantly lower than the HOMO-LUMO gaps of the corresponding unmodified diamondoids.
Efficient preparation of apically substituted diamondoid derivatives. Functionalized Nanodiamonds, part 39. Paul Kahl, Boryslav A. Tkachenko, Anatoliy A. Novikovsky, Jonathan Becker, Jeremy E. P. Dahl, Robert M. K. Carlson, Andrey A. Fokin,* and Peter R. Schreiner* Synthesis 2014, 46,787–798; DOI: 10.1055/s-0033-1338583
We present an effective three-step chromatography-free sequence for the preparation of apical monohydroxy derivatives of diamantane, triamantane, and [121]tetramantane from the corresponding bis-apical diols utilizing tert-butyldimethylsilyl chloride as the monosilylating agent. The procedure was successfully applied to the monoprotection of several other aliphatic and aromatic diols. Additionally, 9-aminodiamantan-4-carboxylic acid, which has significant potential in medicinal and material sciences, was prepared through Ritter reaction of 4,9-dihydroxydiamantane in trifluoroacetic acid.
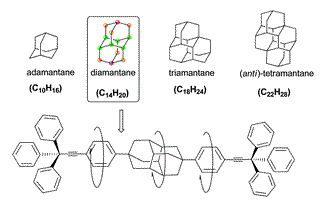
Diamondoids: Functionalization and subsequent applications of perfectly defined molecular cage hydrocarbons. Functionalized Nanodiamonds, part 41. Maria A. Gunawan, Jean-Cyrille Hierso,* Didier Poinsot, Andrey A. Fokin, Natalie A. Fokina, Boryslav A. Tkachenko, and Peter R. Schreiner,* New. J. Chem. 2014, 38, 28–41. DOI: 10.1039/c3nj00535f.
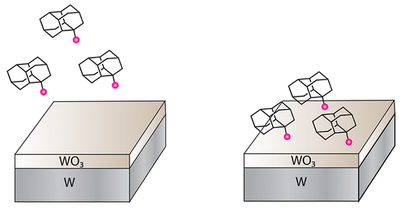
Covalent Attachment of Diamondoid Phosphonic Dichlorides to Tungsten Oxide Surfaces. Functionalized Nanodiamonds, part 40. Fei Hua Li, Jason D. Fabbri, Raisa I. Yurchenko, Alexander N. Mileshkin, James N. Hohmann, Hao Yan, Honyuan Yuan, Ich C. Tran, Trevor Willey, Michael Bagge-Hansen, Jeremy E. P. Dahl, Robert M. K. Carlson, Andrey A. Fokin, Peter R. Schreiner, Zhi-Xun Shen, Nicholas A. Melosh* Langmuir 2013, 29, 9790–9797. DOI: 10.1021/la401781e
Exploring covalently bound diamondoid particles with valence photoelectron spectroscopy. Functionalized Nanodiamonds, part 37. Tobias Zimmermann, Robert Richter, Andre Knecht, Andrey A. Fokin, Tetyana V. Koso, Lesya V. Chernish, Pavel A. Gunchenko, Peter R. Schreiner, Thomas Möller, and Torbjörn Rander
J. Chem. Phys. 2013, 139, 084310-084316. DOI: 10.1063/1.4818994
We investigated the valence electronic structure of diamondoid particles in the gas phase, utilizing valence photoelectron spectroscopy. The samples were singly or doubly covalently bonded dimers or trimers of the lower diamondoids. Both the bond type and the combination of bonding partners are shown to affect the overall electronic structure. For singly bonded particles, we observe a small impact of the bond on the electronic structure, whereas for doubly bonded particles, the connecting bond determines the electronic structure of the highest occupied orbitals. In the singly bonded particles a superposition of the bonding partner orbitals determines the overall electronic structure. The experimental findings are supported by density functional theory computations at the M06-2X/cc-pVDZ level of theory.
The Lipophilic Bullet Hits the Targets: Medicinal Chemistry of Adamantane Derivatives. Functionalized Nanodiamonds, part 27. Lukas Wanka, Khalid Iqbal, and Peter R. Schreiner* Chem. Rev. 2013, 113, 3516–3604. DOI: 10.1021/cr100264t.
Listed as one of the 10 most accessed articles 03/2013.

Evidence of Diamond Nanowires Formed inside Carbon Nanotubes from Diamantane Dicarboxylic Acid. Jinying Zhang, Zhen Zhu, Yanquan Feng, Hitoshi Ishiwata, Yasumitsu Miyata, Ryo Kitaura, Jeremy E. P. Dahl, Robert M. K. Carlson, Natalie A. Fokina, Peter R. Schreiner, David Tomanek, Hisanori Shinohara Angew. Chem. Int. Ed. 2013, 52, 3717–3721. Angew. Chem. 2013, 125, 3805–3809. DOI: 10.1002/anie.201209192. Designated as a “Hot paper”;
Highlights: a) Inside cover of this issue; b) Phys.org, March 6, 2013. c) Chemie.de, March 2013. d) Innovations Report, March 6, 2013. d) TechniScience, March 2013. e) e! Science News, March 6, 2013.
f) GIT Laboratory Journal, March 2013.
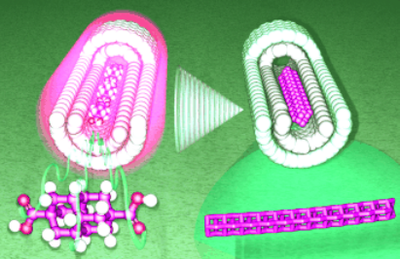
Preparative Synthesis of Vinyl Diamondoids. Functionalized Nanodiamonds, part 36. Andrey A. Fokin,* Ekaterina D. Butova, Anastasiya V. Barabash, Nhan N. Huu, Boryslav A. Tkachenko, and Peter R. Schreiner* Synth. Commun. 2013, 43, 1772–1777.
We describe a convenient four-step preparation of 1- vinyl adamantane, 1- vinyl diamantane, and 4,9-divinyl diamantane from the respective diamondoid acetic acids in 50–80% isolated yields involving esterification, reduction, and hydrobromination/dehydrobromination.
Supplemental materials are available for this article. Go to the publisher's online edition of Synthetic Communications® to view the free supplemental file.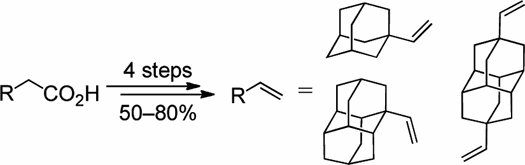
Electronic structure tuning of diamondoids through functionalization. Torbjörn Rander, Matthias Staiger, Robert Richter, Tobias Zimmermann, Lasse Landt, David Wolter, Jeremy E. P. Dahl, Robert M. K. Carlson, Boryslav A. Tkachenko, Natalie A. Fokina, Peter R. Schreiner, Thomas Möller, and Christoph Bostedt J. Chem. Phys. 2013, 135, 024310 (1–7).
We investigated the changes in electronic structures induced by chemical functionalization of the five smallest diamondoids using valence photoelectron spectroscopy. Through the variation of three parameters, namely functional group (thiol, hydroxy, and amino), host cluster size (adamantane, diamantane, triamantane, [121]tetramantane, and [1(2,3)4]pentamantane), and functionalization site (apical and medial) we are able to determine to what degree these affect the electronic structures of the overall systems. We show that unlike, for example, in the case of halobenzenes, the ionization potential does not show a linear dependence on the electronegativity of the functional group. Instead, a linear correlation exists between the HOMO-1 ionization potential and the functional group electronegativity. This is due to localization of the HOMO on the functional group and the HOMO-1 on the diamondoid cage. Density functional theory supports our interpretations.
Photocathode Device using Diamondoid and Cesium Bromide Films. William A. Clay, Juan R. Maldonado, Piero Pianetta, Jeremy E. P. Dahl, Robert M. K. Carlson, Peter R. Schreiner, Andrey A. Fokin, Boryslav A. Tkachenko, Nicolas A. Melosh, Zhi-Xun Shen,
Appl. Phys. Lett. 2012, 101, 241605/1–5. DOI: 10.1063/1.4769043.
A photocathode structure is presented that shows promise for use in high brightness electron sources. The structure consists of a metal substrate, a monolayer of a diamondoid derivative, and a thin film of cesium bromide. Diamondoid monolayers reduce the energy spread of electron emitters, while cesium bromide increases the yield and stability of cathodes. We demonstrate that the combined structure retains these properties, producing an emitter with lower energy spread than the corresponding cesium bromide emitter (1.06 eV versus 1.45 eV) and higher yield and stability than un-coated diamondoid emitters.
Diamondoid coating enables disruptive approach for chemical and magnetic imaging with 10 nm spatial resolution. Hitoshi Ishiwata, Yves Acremann, Andreas Scholl, Olav Hellwig, Elisabeth Dobisz, Andrew Doran, Boryslav A. Tkachenko, Andrey A. Fokin, Peter R. Schreiner, Jeremy E. P. Dahl, Robert M. K. Carlson, Nicolas A. Melosh, Zhi-Xun Shen, Hendrik Ohldag Appl. Phys. Lett. 2012, 101, 163101. DOI: 10.1063/1.4756893.
Diamondoids are unique molecular nano-materials with diamond structure and fascinating properties such as negative electron affinity and short electron mean free paths. A thin layer of diamondoids deposited on a cathode is able to act as an electron monochromator, reducing the energy spread of photo-emitted electrons from a surface. This property can be applied effectively to improve the spatial resolution in x-ray photoemission electron microscopy (X-PEEM), which is limited by chromatic aberration of the electron optics. In this paper, we present X-PEEM measurements reaching the technological relevant spatial resolution of 10 nm without the need of expensive and complex corrective optics. Our results provide a simple approach to image surface chemical and magnetic information at nanometer scales by employing diamondoids.
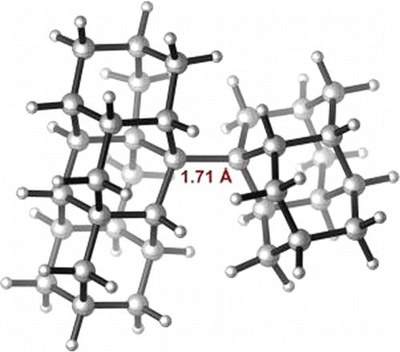
Stable Alkanes Containing Very Long Carbon-Carbon Bonds. Andrey A. Fokin, Lesya V. Chernish, Pavel A. Gunchenko, Evgeniya Yu. Tikhonchuk, Heike Hausmann, Michael Serafin, Jeremy E. P. Dahl, Robert M. K. Carlson, and Peter R. Schreiner
J. Am. Chem. Soc. 2012, 134, 13641–13650. DOI: 10.1021/ja302258q.
Nanodiamonds in Sugar Rings: An Experimental and Theoretical Investigation of Cyclodextrin-Nanodiamond Inclusion Complexes. Functionalized Nanodiamonds, part 31. Jens Voskuhl, Mark Waller, Sateesh Bandaru, Boryslav A. Tkachenko, Carlo Fregonese, Birgit Wibbeling, Peter R. Schreiner,* and Bart Jan Ravoo*
Org. Biomol. Chem. 2012, 10, 4524–4530. Highlight: Inside cover of this issue.
We report on the noncovalent interactions of nanodiamond carboxylic acids derived from adamantane, diamantane, and triamantane with β- and γ-cyclodextrins. The water solubility of the nanodiamonds was increased by attaching an aromatic dicarboxylic acid via peptide coupling. Isothermal titration calorimetry experiments were performed to determine the thermodynamic parameters (Ka, ΔH, ΔG and ΔS) for the host–guest inclusion. The stoichiometry of the complexes is invariably 1 : 1. It was found that Ka, ΔG and ΔH of inclusion increase for larger nanodiamonds. ΔS is generally positive, in particular for the largest nanodiamonds. β-Cyclodextrin binds all nanodiamonds, γ-cyclodextrin clearly prefers the most bulky nanodiamonds. The interaction of 9-triamantane carboxylic acid shows one of the strongest complexation constants towards γ-cyclodextrin ever reported, Ka = 5.0 × 105 M−1. In order to gain some insight into the possible structural basis of these inclusion complexes we performed density functional calculations at the B97-D3/def2-TZVPP level of theory.
Synthesis of Diamondoid Carboxylic Acids. Functionalized Nanodiamonds, part 30. Natalie A. Fokina, Boryslav A. Tkachenko, Jeremy E. P. Dahl, Robert M. K. Carlson, Andrey A. Fokin, and Peter R. Schreiner* Synthesis 2012, 44, 259–264.
Procedures for the synthesis of apical mono-, di-, and tricarboxylic acids of triamantane, [121]tetramantane, and [1(2,3)4]pentamantane, apical diacetic acids of diamantane and triamantane, as well as medial propionic acids of diamantane and triamantane were elaborated starting from the corresponding alcohols and bromides. The obtained diamondoid acids were characterized as their methyl ester derivatives.
Overcoming Extremely Long C–C Alkane Bond Lability through Attractive Dispersion Forces. Functionalized Nanodiamonds, part 29. Peter R. Schreiner,* Lesya V. Chernish, Pavel A. Gunchenko, Evgeniya Yu. Tikhonchuk, Heike Hausmann, Michael Serafin, Sabine Schlecht, Jeremy E. P. Dahl, Robert M. K. Carlson, and Andrey A. Fokin* Nature 2011, 477, 308–311.
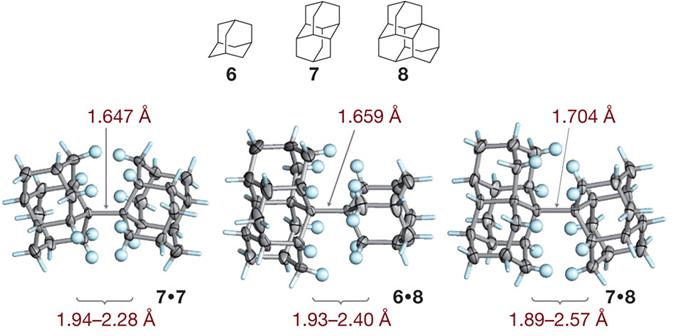
Steric effects in chemistry are a consequence of the space required to accommodate the atoms and groups within a molecule, and are often thought to be dominated by repulsive forces arising from overlapping electron densities (Pauli repulsion). An appreciation of attractive interactions such as van der Waals forces (which include London dispersion forces) is necessary to understand chemical bonding and reactivity fully. This is evident from, for example, the strongly debated origin of the higher stability of branched alkanes relative to linear alkanes and the possibility of constructing hydrocarbons with extraordinarily long C-C single bonds through steric crowding. Although empirical bond distance/bond strength relationships have been established for C-C bonds (longer C-C bonds have smaller bond dissociation energies), these have no present theoretical basis. Nevertheless, these empirical considerations are fundamental to structural and energetic evaluations in chemistry, as summarized by Pauling as early as 1960 and confirmed more recently. Here we report the preparation of hydrocarbons with extremely long C-C bonds (up to 1.704 Å), the longest such bonds observed so far in alkanes. The prepared compounds are unexpectedly stable--noticeable decomposition occurs only above 200 °C. We prepared the alkanes by coupling nanometre-sized, diamond-like, highly rigid structures known as diamondoids. The extraordinary stability of the coupling products is due to overall attractive dispersion interactions between the intramolecular H•••H contact surfaces, as is evident from density functional theory computations with and without inclusion of dispersion corrections.
Diamondoid-modified DNA. Functionalized Nanodiamonds, part 28.
Yan Wang, Boryslav A. Tkachenko, Peter R. Schreiner,* and Andreas Marx*
Org. Biomol. Chem. 2011, 9, 7482–7490.
 We prepared novel C5-modified triphosphates and phosphoramidites with a diamondoid functionally linked to the nucleobase. Using primer extension experiments with different length templates we investigated whether the modified triphosphates were enzymatically incorporated into DNA and whether they were further extended. We found that all three modified nucleotides can be incorporated into DNA using a single-nucleotide incorporation experiment, but only partially using two templates that demand for multiple incorporation of the modified nucleotides. The modified phosphoramidites were introduced into oligonucleotides utilizing DNA synthesizer technology. The occurring oligonucleotide structures were examined by circular dichroism (CD) and melting temperature (Tm) measurements and were found to adapt similar helix conformations as their unmodified counterparts.
We prepared novel C5-modified triphosphates and phosphoramidites with a diamondoid functionally linked to the nucleobase. Using primer extension experiments with different length templates we investigated whether the modified triphosphates were enzymatically incorporated into DNA and whether they were further extended. We found that all three modified nucleotides can be incorporated into DNA using a single-nucleotide incorporation experiment, but only partially using two templates that demand for multiple incorporation of the modified nucleotides. The modified phosphoramidites were introduced into oligonucleotides utilizing DNA synthesizer technology. The occurring oligonucleotide structures were examined by circular dichroism (CD) and melting temperature (Tm) measurements and were found to adapt similar helix conformations as their unmodified counterparts.Synthesis of Higher Diamondoids and Implications of Their Formation in Petroleum. Jeremy E. P. Dahl,* J. Michael Moldovan, Zhibin Wei, Paul A. Lipton, Peter Denisevich, Shengao Liu, Peter R. Schreiner,* and Robert M. K. Carlson Angew. Chem. Int. Ed. 2011, 49, 9881–9885.
Could chemistry be as easy as A + B = C? En route to laboratory diamond synthesis: We show that higher diamondoids form from lower ones in experiments mimicking petroleum cracking. The yields are low but can be significantly improved by the addition of isobutane or isobutene. Rather than through superacid-catalyzed carbocation rearrangement reactions – long assumed to be responsible for diamondoid growth – our experiments take place via free-radical mechanisms that are akin to CVD growth giving microcrystalline diamond. Indeed, we used higher diamondoids as seeds to grow CVD diamond, and it should be possible to synthesize larger nanodiamonds and perhaps even diamond using diamondoids as seeds.
Negative-electron-affinity diamondoid monolayers as high-brilliance source for ultrashort electron pulses. S. Roth, D. Leuenberger, J. Osterwalder, J.E. Dahl, R.M.K. Carlson, B.A. Tkachenko, A.A. Fokin, P.R. Schreiner, M. Hengsberger Chem. Phys. Lett. 2010, 495, 102-108.
Diamondoids are nanometer-sized, hydrogen terminated diamond-like molecules consisting of fused adamantane units. The thiolated diamondoid [121]tetramantane-6-thiol shows negative electron affinity behavior, i.e. population of unoccupied states directly leads to spontaneous electron emission. We present time-resolved photoemission data from self-assembled monolayers of [121]tetramantane-6-thiol in order to shed light on the emission process: A photon energy threshold for electron emission of 5.6–5.8 eV was observed, and the electron affinity was estimated to be -0.21 to -0.57 eV for Ag and Au substrates, respectively. Electrons are emitted after excitation in the metal substrate through the molecular orbitals within a few femtoseconds. 
Molybdenum(VI) Oxide-Organic Frameworks (MOOFs), a New Series of Coordination Hybrids Constructed with Bitopic 1,2,4-Trialzole Linkers. Andrey B. Lysenko, Ganna A. Senchyk, Jörg Lincke, Daniel Lässig, Andrey A. Fokin, Peter R. Schreiner, Harald Krautscheid, and Konstantin V. Domasevitch Dalton Trans. 2010, 39, 4223–4231. A series of molybdenum(VI) oxide-organic solids were prepared by hydrothermal reactions employing N-donor tectons, which combine two 1,2,4-triazol-4-yl sites separated by representative aliphatic spacers (ethylene, tr2eth; 1,3-propylene, tr2pr; trans-1,4-cyclohexanediyl, tr2cy; diamondoid 1,3-adamantanediyl, tr2ad; 1,6- and 4,9-diamantanediyls, 1,6-tr2dia and 4,9-tr2dia) and heterofunctional 5-[4-(1,2,4-triazol-4-yl)phenyl]tetrazole (trtz). In all the compounds the 1,2,4-triazol-4-yl group acts as a short-distance N1,N2-bridge between two Mo ions (Mo-N 2.36–2.50 Å). The 3D framework structure is based upon pseudo-41 helices ([Mo4O12(tr2eth)2] 1, [Mo2O6(tr2cy)] 3) or sinusoidal chains ([Mo2O6(tr2pr)] 2, [Mo2O6(tr2ad)]•6H2O 4, [Mo2O6(4,9-tr2dia)]•0.5H2O 5) of vertex-sharing MoO4N2 octahedra, while the bitopic organic ligands manifest a dual role as connectors for two adjacent octahedra and as links between separate 1D inorganic subtopologies. For linear, lengthy tectons tr2cy and 4,9-tr2dia two identical frameworks interpenetrate. In [MoO3(trtz)] 7, the 41 helices aggregate into 3D tetragonal framework (five-fold interpenetrated) by hydrogen bonding between the tetrazole groups. 2D structure of [Mo4O12(1,6-tr2dia)]•2H2O 6 is influenced by very bulk aliphatic portion of the ligands, which connect chains of edge-shared octahedra MoO5N (Mo-N 2.346(2), 2.456(2) Å).
A series of molybdenum(VI) oxide-organic solids were prepared by hydrothermal reactions employing N-donor tectons, which combine two 1,2,4-triazol-4-yl sites separated by representative aliphatic spacers (ethylene, tr2eth; 1,3-propylene, tr2pr; trans-1,4-cyclohexanediyl, tr2cy; diamondoid 1,3-adamantanediyl, tr2ad; 1,6- and 4,9-diamantanediyls, 1,6-tr2dia and 4,9-tr2dia) and heterofunctional 5-[4-(1,2,4-triazol-4-yl)phenyl]tetrazole (trtz). In all the compounds the 1,2,4-triazol-4-yl group acts as a short-distance N1,N2-bridge between two Mo ions (Mo-N 2.36–2.50 Å). The 3D framework structure is based upon pseudo-41 helices ([Mo4O12(tr2eth)2] 1, [Mo2O6(tr2cy)] 3) or sinusoidal chains ([Mo2O6(tr2pr)] 2, [Mo2O6(tr2ad)]•6H2O 4, [Mo2O6(4,9-tr2dia)]•0.5H2O 5) of vertex-sharing MoO4N2 octahedra, while the bitopic organic ligands manifest a dual role as connectors for two adjacent octahedra and as links between separate 1D inorganic subtopologies. For linear, lengthy tectons tr2cy and 4,9-tr2dia two identical frameworks interpenetrate. In [MoO3(trtz)] 7, the 41 helices aggregate into 3D tetragonal framework (five-fold interpenetrated) by hydrogen bonding between the tetrazole groups. 2D structure of [Mo4O12(1,6-tr2dia)]•2H2O 6 is influenced by very bulk aliphatic portion of the ligands, which connect chains of edge-shared octahedra MoO5N (Mo-N 2.346(2), 2.456(2) Å).Experimental and Theoretical Studies of the Absorption Properties of Thiolated Diamondoids. Lasse Landt, Christoph Bostedt, Thomas Möller, Roland Mitric, Jeremy E. P. Dahl, Robert M. K. Carlson, Boryslav A. Tkachenko, Andrey A. Fokin, Peter R. Schreiner, Alexander Kulesza, and Vlasta Bonacic-Koutecky J. Chem. Phys. 2010, 132, 144305.
Nanoscale hybrid systems are a new class of molecular aggregates that offer numerous new possibilities in materials design. Diamondoid thiols are promising nanoscale building blocks for such hybrid systems. They allow the incorporation of functional groups and the investigation of their effects on the unique materials’ properties of diamondoids. Here we combine experimental data with ab initio theory to explore the optical properties of diamondoid thiols and their dependence on size and shape. Agreement between theoretically and experimentally obtained absorption spectra allows the identification of the nature of the optical transitions that are responsible for some photophysical and photochemical processes. We show that the optical properties of diamondoid thiols in the deep UV regime depend on the functionalization site but are largely size independent. Our findings provide an explanation for the disappearance of diamondoid UV photoluminescence upon thiolation for smaller diamondoids. However, our theoretical results indicate that for larger diamondoid thiols beyond the critical size of six diamondoid cages the lowest energy transitions are characterized by diamondoidlike states suggesting that UV luminescence may be regained.
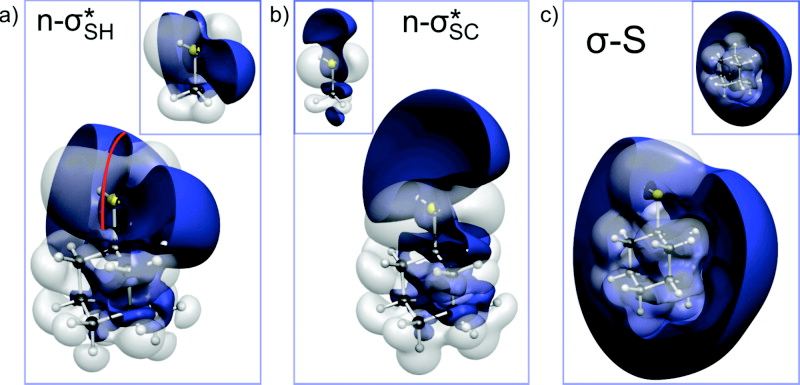
Diamondoid Phosphines – Selective Phosphorylation of Nanodiamonds. Hartmut Schwertfeger, Mareike Machuy, Christian Würtele, Jeremy E. P. Dahl, Robert M. K. Carlson, Andrey A. Fokin, and Peter R. Schreiner Adv. Synth. Cat. 2010, 352, 609–615.
The diamondoids (nanodiamonds) diamantane and triamantane were selectively converted into diorganophosphinic acid chlorides by reacting them with phosphorus trichloride under Friedel-Crafts-like conditions. The di-diamondoid phosphinic acid chlorides were subsequently reduced with trichlorosilane to give the hitherto unknown corresponding di-diamondoid phosphines. These diamondoid phosphinic acid chlorides and phosphines are of great utility as starting materials in organo-element and coordination chemistry due to their extraordinary rigidity and steric bulk. Synthesis of Diamondoid Nitro Compounds from Amines with m-Chlorobenzoic Acid. Hartmut Schwertfeger, Christian Würtele, and Peter R. Schreiner
Synlett 2010, 493–495.
We report the synthesis of diamondoid nitro derivatives via the selective oxidation of the corresponding amines using m-chloroperbenzoic acid in 1,2-dichloroethane as solvent.
Synthetic Routes to Aminotriamantanes, Topological Analogues of the Neuroprotector Memantine. Andrey A. Fokin, Anika Merz, Natalie A. Fokina, Hartmut Schwertfeger, Shenggao L. Liu, Jeremy E. P. Dahl, Robert M. K. Carlson, and Peter R. Schreiner
Synthesis 2009, 909–912.
The amino derivatives of diamantane and triamantane representing close topological analogues of the neuroprotective drug memantine were prepared via amination of the respective carboxylic acids or alcohols. Synthesis of Diamantane-Derived N-Heterocyclic Carbenes (IDAd) and Applications in Catalysis. Heinrich Richter, Hartmut Schwertfeger, Peter R. Schreiner, Roland Fröhlich, and Frank Glorius Synlett 2009, 193–197.
Novel diamantyl-substituted imidazolium salts have been synthesized, characterized and, in addition, analyzed by single crystal structural analysis. The corresponding NHCs (a-IDAd and m-IDAd) have been prepared in solution and have been characterized by NMR. They exhibit increased steric demand and increased lipophilicity relative to the well-known IAd. In comparative studies, these NHCs were tested as ligands in palladium-catalyzed Sonogashira reactions of primary alkyl halides and, in addition, as catalysts in an organocatalyzed silyl enol ether formation. 
[123]Tetramantane: Parent of a New Family of alpha-Helicenes. Peter R. Schreiner, Andrey A. Fokin, Boryslav A. Tkachenko, Elemer Vass, Marylin Olmstead, Dieter Bläser, Roland Boese, Hans Peter Reisenauer, Jeremy E. P. Dahl, and Robert M. K. Carlson J. Am. Chem. Soc. 2009, 131, 11292–11293.
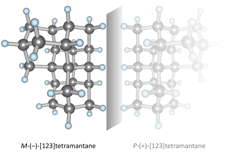
We present a new type of alpha-helical structure based on a diamondoid (nanodiamond) framework, C2-symmetric [123]tetramantane, whose (+) and (–) isomers could be enantioseparated by HPLC techniques. Bromination of the enantiopure hydrocarbon led to the isolation of (+)-7-bromo-[123]tetramantane, which could be crystallized and subjected to X-ray structure analysis. By using the anomalous dispersion, we identify this compound as the P-isomer for the hydrocarbon moiety. Experimental as well as computed ORD and VCD spectra independently and in agreement with the X-ray structure analysis give M-(–) for the configuration of the second eluted parent hydrocarbon isomer. Photoacetylation of Diamondoids: Selectivities and Mechanism. Andrey A. Fokin, Pavel A. Gunchenko, Anatoliy A. Novikovsky, Tatyana E. Shubina, Boris V. Chernyaev, Jeremy E. P. Dahl, Robert M. K. Carlson, Alexander G. Yurchenko, and Peter R. SchreinerEur. J. Org. Chem. 2009, 5153–5161.
The cover picture shows the photochemical generation of triplet diacetyl (butadione, center) and its highly selective C–H bond activation reactions in the functionalizations of tertiary C–H bonds. Despite several similarly reactive tertiary C–H bonds and a multitude of secondary C–H bonds with similar or even smaller bond dissociation energies (blue hydrogens on structures on the left), this reagent shows a remarkable selectivity for the apical positions of diamondoids owing to high sensitivity to steric hindrance. This gives the respective apical acetyl diamondoids (blue groups on the right) with high yields. Deuterium kinetic isotope effects and accompanying computational studies shed light on the rate-determining C–H bond activation step.
Oxygen-Doped Nanodiamonds: Synthesis and Functionalizations. Andrey A. Fokin, Tatyana S. Zhuk, Alexander E. Pashenko, Pavlo O. Dral, Pavel A. Gunchenko, Jeremy E. P. Dahl, Robert M. K. Carlson, Tatyana V. Koso, Michael Serafin, and Peter R. SchreinerOrg. Lett. 2009, 11, 3068–3071.
Oxadiamondoids representing a new class of carbon nanoparticles were prepared from the respective diamondoid ketones via an effective two-step procedure involving addition of methyl magnesium iodide and oxidation with trifluoroperacetic acid in trifluoroacetic acid. The reactivities of the oxacages are determined by the position of the dopant and are in good agreement with computational predictions.
Band Gap Tuning in Nanodiamonds: First Principle Computational Studies. Andrey A. Fokin and Peter R. Schreiner Mol. Phys. 2009, 107, 823–830.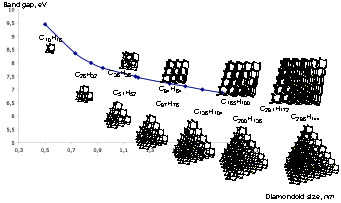
We present a density functional theory study on changes in band gap effects of nanodiamonds (hydrogen terminated diamond-like molecules, diamondoids) depending on size, shape, and the incorporation of heteroatom functionalities. Strong quantum confinement effects were identified at particle sizes from 0.5 to at least 2 nm, when the band gaps of these nanodiamonds are reduced to 6.7 eV. Octahedral and tetrahedral nanodiamonds show the same trends in band gap narrowing, and it is the dimension rather than the shape/morphology of the nanodiamonds that alters the band gaps. Band gap tuning through external (by C–H bond substitution) or internal (by replacing CH or CH2 moieties) doping is non-additive for the same dopant. Push-pull doping, with electron donating and electron withdrawing groups is most effective and reduces the band gaps of diamondoids to that of bulk diamond. Further reductions down to 1–2 eV are conceivable with charged external substituents. The combination of increasing the size of the nanodiamond and push-pull doping are likely to make these materials highly valuable for semiconductor applications. Selective Preparation of Diamondoid Fluorides. Harmut Schwertfeger, Christian Würtele, Heike Hausmann, Jeremy E. P. Dahl, Robert M. K. Carlson, Andrey A. Fokin, and Peter R. Schreiner Adv. Synth. Cat. 2009, 351, 1041–1054.The selective fluorination of diamantane, triamantane, [121]tetramantane, and [1(2,3)4]pentamantane bromides and alcohols was achieved by using the fluorinating agents AgF and DAST. Various mono-, di-, tri- and even tetrafluorinated diamondoid derivatives were prepared and characterized. We were also able to prepare the amino fluoro and the fluoro alcohol of diamantane from the corresponding monoprotected diamondoid diols. These reactions can be carried out in a highly selective manner and proceed without isomerizations. The fluorinated, unequally disubstituted derivatives are valuable compounds for the exploration of electronic, pharmacological, and material properties of functionalized diamondoids.  Synthesis, Characterization, and Property Evaluations of Copolymers of Diamantyl Methacrylate with Methyl Methacrylate. Carsten Sinkel, Seema Agarwal, Natalie A. Fokina, Peter R. Schreiner J. Appl. Polym. Sci. 2009, 114, 2109–2115.
Synthesis, Characterization, and Property Evaluations of Copolymers of Diamantyl Methacrylate with Methyl Methacrylate. Carsten Sinkel, Seema Agarwal, Natalie A. Fokina, Peter R. Schreiner J. Appl. Polym. Sci. 2009, 114, 2109–2115.
This work reports the homo- and copolymerization behavior of previously unknown 4-diamantyl methacrylate (DMA) with vinyl comonomers like methyl methacrylate (MMA);the starting monomer DMA was synthesized in our laboratory using 4-diamantanol and methacryloylchloride. The structures of the homo- and copolymers, were analyzed using NMR techniques. DMA was found to be more reactive than MMA during copolymerization. The reactivity ratios as determined by the Kelen-Tüdos method are rMMA = 0.58 and rDMA = 1.75. The incorporation of a few mol% of diamantyl units into the PMMA backbone led to an increase in its thermal stability and glass transition temperature. The polymers with 15 and 42 mol% of DMA units in the copolymers showed increased Tg values of 126 °C and 172 °C, respectively. Determining Orientational Structure of Diamondoid Thiols Attached to Silver Using Near-Edge X-ray Absorption Fine Structure Spectroscopy. Trevor M. Willey, Jonathan R. I. Lee, Jason D. Fabbri, Dongbo Wang, Michael H. Nielsen, Jason C. Randel, Peter R. Schreiner, Andrey A. Fokin, Boryslav A. Tkachenko, Natalyia A. Fokina, Jeremy E. P. Dahl, Robert M. K. Carlson, Louis J. Terminello, Nicolas A. Melosh, and Tony van Buuren J. Elec. Spec. Rel. Phenom. 2009, 172, 69–77.Near-edge X-ray absorption fine structure spectroscopy (NEXAFS) is a powerful tool for determination of molecular orientation in self-assembled monolayers and other surface-attached molecules. A general framework for using NEXAFS to simultaneously determine molecular tilt and twist of rigid molecules attached to surfaces is presented. This framework is applied to self-assembled monolayers of higher diamondoids, hydrocarbon molecules with cubic-diamond-cage structures. Diamondoid monolayers chemisorbed on metal substrates are known to exhibit interesting electronic and surface properties. This work compares molecular orientation in monolayers prepared on silver substrates using two different thiol positional isomers of [121]tetramantane, and thiols derived from two different pentamantane structural isomers, [1212]pentamantane and [1(2,3)4]pentamantane. The observed differences in monolayer structure demonstrate the utility and limitations of NEXAFS spectroscopy and the framework. The results also demonstrate the ability to control diamondoid assembly, in particular the molecular orientational structure, providing a flexible platform for the modification of surface properties with this exciting new class of nanodiamond materials. 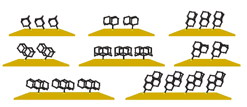
Origin of the Monochromatic Photoemission Peak in Diamondoid Monolayers. W. A. Clay, Z. Liu, W. Yang, J. D. Fabbri, J. E. Dahl, R. M. K. Carlson, Y. Sun, P. R. Schreiner, A. A. Fokin, B. A. Tkachenko, N. A. Fokina, P. A. Pianetta, N. Melosh, and Z.-X. ShenNano Lett. 2009, 9, 57-61.Abstract. Recent photoemission experiments have discovered a highly monochromatized secondary electron peak emitted from diamondoid self-assembled monolayers on metal substrates. New experimental data and simulation results are presented to show that a combination of negative electron affinity and strong electron−phonon scattering is responsible for this behavior. The simulation results are generated using a simple Monte Carlo transport algorithm. The simulated spectra recreate the main spectral features of the measured ones.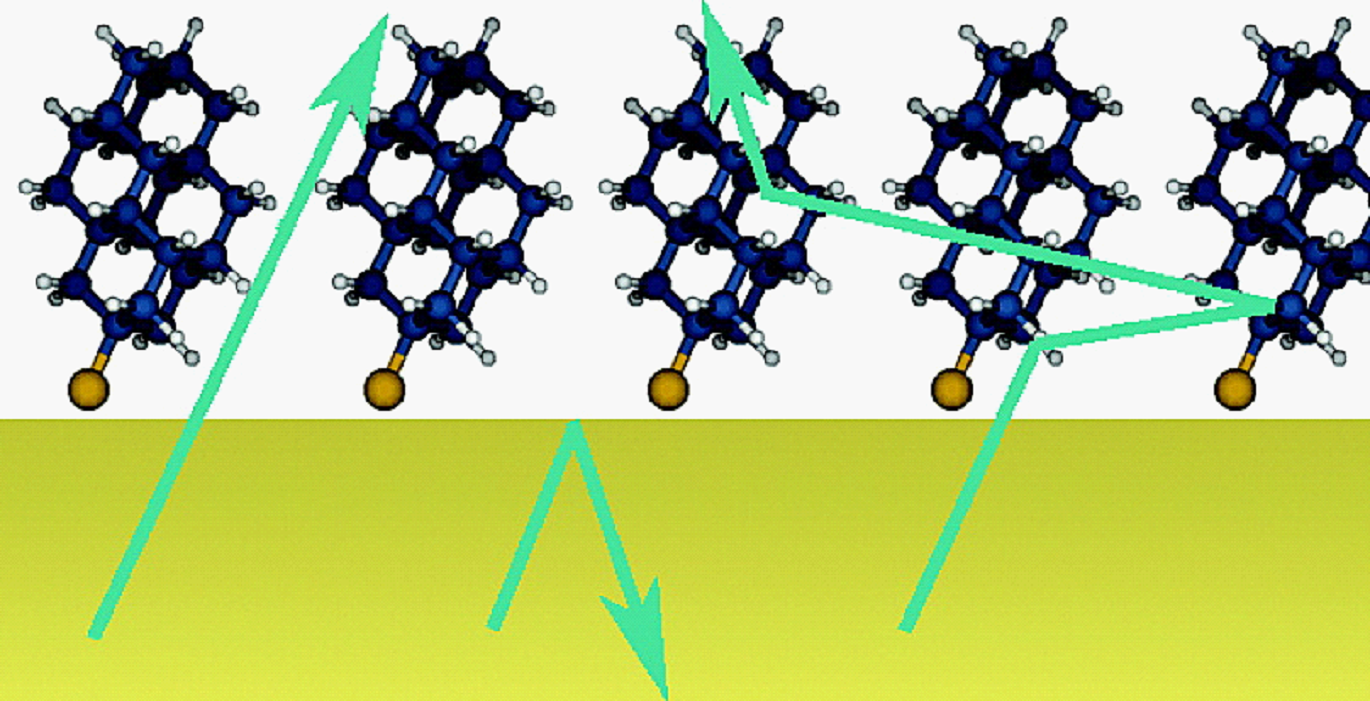
Reactivities of the Prism-Shaped Diamondoids [1(2)3]Tetramantane and [12312]Hexamantane (Cyclohexamantane). A. A. Fokin, B. A. Tkachenko, N. A. Fokina, H. Hausmann, M. Serafin, J. E. P. Dahl, R. M. K. Carlson, P. R. Schreiner
Chem. Eur. J. 2009, 15, 3851-3862.
Abstract. Various functional groups have been incorporated into the structures of the naturally occurring diamondoids [1(2)3]tetramantane and [12312]hexamantane (cyclohexamantane), which represent hydrogen-terminated prism-shaped nanodiamonds. The selectivities of the C—H substitutions in [1(2)3]tetramantane depend on the reagent employed and give products substituted at either central (through bromination) or peripheral (through nitroxylation and photo-oxidation) positions. The hydrogen-coupled electron-transfer mechanism of C—H nitroxylation with the model electrophile NO2+···HNO3 was verified computationally at the B3PW91 and MP2 levels of theory by utilizing the 6-31G(d) and cc-pVDZ basis sets. The thermodynamically controlled nitroxylation/isomerization of [1(2)3]tetramantane allows the preparation of peripherally trisubstituted derivatives, which were transformed into tripod-like nanodiamond building blocks. The bromination of cyclohexamantane selectively gives the 2-bromo derivative, reproducing the chemical behavior of the {111} surface of the hydrogen-terminated diamond. 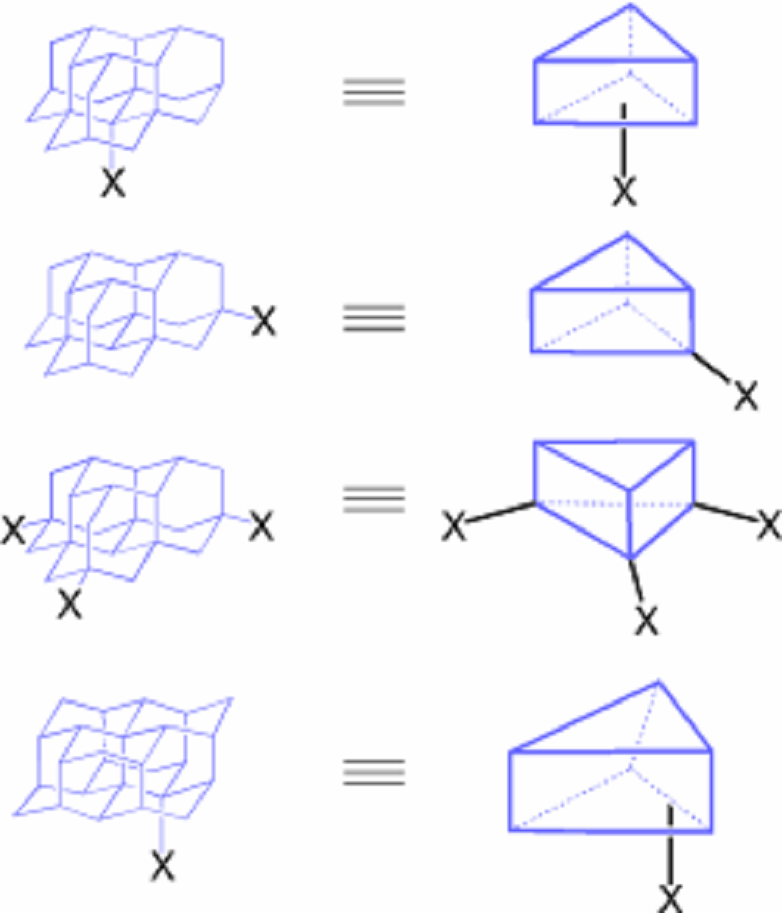
Diamonds are a chemist’s best friend: Diamondoid chemistry beyond adamantane. Hartmut Schwertfeger, Andrey A. Fokin, and Peter R. SchreinerAngew. Chem. Int. Ed. 2008, 47, 1022-1036. Listed as one of the most accessed articles 2008.
Abstract. Marilyn Monroe knew that “diamonds are a girl's best friend” but, in the meantime, many chemists have realized that they are also extremely attractive objects in contemporary chemistry. The chemist's diamonds are usually quite small (herein: nanometer-sized “diamondoids”) and as a result of their unique structure are unusual chemical building blocks. Since lower diamondoids (up to triamantane) have recently become available in large amounts from petroleum and higher diamondoids (starting from tetramantane) are now also accessible from crude oil new research involving them has begun to emerge. Having well-defined structures makes these cage compounds so special compared to other nanometer-scale diamonds. Selective and high-yielding synthetic approaches to the functionalization of diamondoids gives derivatives that can find applications in, for example, polymers, coating materials, drugs, and molecular electronics. Monoprotection of Diols as a Key Step for the Selective Synthesis of Unequally Disubstituted Diamondoids (Nanodiamonds). Hartmut Schwertfeger, Christian Würtele, Michael Serafin, Heike Hausmann, Robert M. K. Carlson, Jeremy E. P. Dahl, and Peter R. Schreiner J. Org. Chem. 2008, 73, 7789-7792;Highlight: Monoprotection of Diamondoid Diols. Timothy M. Swager, Eric L. Dane Synfacts 2008, 1275.
Monoprotection of Diols as a Key Step for the Selective Synthesis of Unequally Disubstituted Diamondoids (Nanodiamonds). Hartmut Schwertfeger, Christian Würtele, Michael Serafin, Heike Hausmann, Robert M. K. Carlson, Jeremy E. P. Dahl, and Peter R. Schreiner J. Org. Chem. 2008, 73, 7789-7792;Highlight: Monoprotection of Diamondoid Diols. Timothy M. Swager, Eric L. Dane Synfacts 2008, 1275.
Abstract. The monoprotection (desymmetrization) of diamondoid, benzylic, and ethynyl diols has been achieved using fluorinated alcohols such as 2,2,2-trifluoroethanol (TFE) under acidic conditions. This practical acid-catalyzed SN1 reaction opens the door for the synthesis of novel bifunctional diamondoids. With diamantane as an example, we show that the resulting monoethers can be used to prepare selectively, for instance, amino or nitro alcohols and unnatural amino acids. These are important compounds in terms of the exploration of electronic, pharmacological, and material properties of functionalized nanodiamonds.
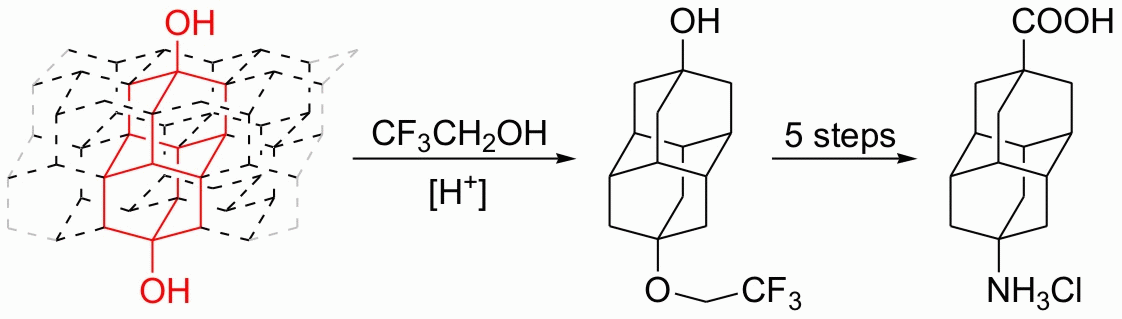
Monochromatic Electron Emission from Diamondoid Monolayers. W. K. Yang, J. D. Fabbri, T. M. Willey, J. R. I. Lee, J. E. P. Dahl, R. M. K. Carlson, P. R. Schreiner, A. A. Fokin, B. A. Tkachenko, N. A. Fokina, W. Meevasana, N. Mannella, K. Tanaka, X. J. Zhou, T. van Buuren, M. A. Kelly, Z. Hussain, N. A. Melosh, Z.-X. Shen Science 2007, 316, 1460-1462;Abstract. We found monochromatic electron photoemission from large-areaself-assembled monolayers of a functionalized diamondoid, [121]tetramantane-6-thiol.Photoelectron spectra of the diamondoid monolayers exhibiteda peak at the low–kinetic energy threshold; up to 68%of all emitted electrons were emitted within this single energypeak. The intensity of the emission peak is indicative of diamondoidsbeing negative electron affinity materials. With an energy distributionwidth of less than 0.5 electron volts, this source of monochromaticelectrons may find application in technologies such as electronmicroscopy, electron beam lithography, and field-emission flat-paneldisplays. 
HH


