Tunneling Studies
Current work:
Equilibrating Parent Aminomercaptocarbene with CO2 via Heavy-Atom Quantum Tunneling. Bastian Bernhardt, Markus Schauermann, Ephrath Solel, André K. Eckhardt, and Peter R. Schreiner
Sci. 2022, 14, 130–135. DOI: 10.1039/D2SC05388H.
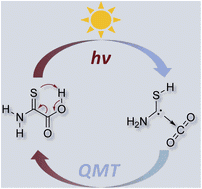
The search for methods to bind CO2 and use it synthetically as a C1-building block under mild conditions is an ongoing endeavor of great urgency. The formation of heterocyclic carbene–carbon dioxide adducts occurs rapidly when the carbene is generated in solution in the presence of CO2. Here we demonstrate the reversible formation of a complex of the hitherto unreported aminomercaptocarbene (H2N–![[C with combining umlaut]](https://www.rsc.org/images/entities/char_0043_0308.gif) –SH) with CO2 isolated in solid argon by photolysis of 2-amino-2-thioxoacetic acid. Remarkably, the complex disappears in the dark as deduced by time-dependent matrix infrared measurements, and equilibrates back to the covalently bound starting material. This kinetically excluded process below ca. 8 K is made possible through heavy-atom quantum mechanical tunneling, as also evident from density functional theory and ab initio computations at the CCSD(T)/cc-pVTZ level of theory. Our results provide insight into CO2 activation using a carbene and emphasize the role of quantum mechanical tunneling in organic processes, even involving heavy atoms.
–SH) with CO2 isolated in solid argon by photolysis of 2-amino-2-thioxoacetic acid. Remarkably, the complex disappears in the dark as deduced by time-dependent matrix infrared measurements, and equilibrates back to the covalently bound starting material. This kinetically excluded process below ca. 8 K is made possible through heavy-atom quantum mechanical tunneling, as also evident from density functional theory and ab initio computations at the CCSD(T)/cc-pVTZ level of theory. Our results provide insight into CO2 activation using a carbene and emphasize the role of quantum mechanical tunneling in organic processes, even involving heavy atoms.
Switching on H-Tunneling Through Conformational Control. José P. L. Roque, Cláudio M. Nunes, Luís P. Viegas, Nelson A. M. Pereira, Teresa M. D. V. Pinho e Melo, Peter R. Schreiner and Rui Fausto
J. Am. Chem. Soc. 2021, 143, 8266-8271. DOI: 10.1021/jacs.1c04329
H-tunneling is a ubiquitous phenomenon, relevant to fields from biochemistry to materials science, but harnessing it for mastering the manipulation of chemical structures still remains nearly illusory. Here, we demonstrate how to switch on H-tunneling by conformational control using external radiation. This is outlined with a triplet 2-hydroxyphenylnitrene generated in an N2 matrix at 10 K by UV-irradiation of an azide precursor. The anti-orientation of the nitrene's OH moiety was converted to syn by selective vibrational excitation at the 2ν(OH) frequency, thereby moving the H atom closer to the vicinal nitrene center. This triggers spontaneous H-tunneling to a singlet 6-imino-2,4-cyclohexadienone. Computations reveal that such fast H-tunneling occurs through crossing the triplet-to-singlet potential energy surfaces. Our experimental realization provides an exciting novel strategy to attain control over tunneling, opening new avenues for directing chemical transformations.
Ethynylhydroxycarbene (H–C≡C–C̈–OH). Bastian Bernhardt, Marcel Ruth, André K. Eckhardt and Peter R. Schreiner
J. Am. Chem. Soc. 2021, 143, 3741–3746. DOI: 10.1021/jacs.1c00897. Highlight: Featured in Chemviews March 08, 2021.
The species on the C3H2O potential energy surface have long been known to play a vital role in extraterrestrial chemistry. Here we report on the hitherto uncharacterized isomer ethynylhydroxycarbene (H-C≡C-C̈-OH, 1) generated by high-vacuum flash pyrolysis of ethynylglyoxylic acid ethyl ester and trapped in solid argon matrices at 3 and 20 K. Upon irradiation at 436 nm trans-1 rearranges to its higher lying cis-conformer. Prolonged irradiation leads to the formation of propynal. When the matrix is kept in the dark, 1 reacts within a half-life of ca. 70 h to propynal in a conformer-specific [1,2]H-tunneling process. Our results are fully consistent with computations at the CCSD(T)/cc-pVTZ and the B3LYP/def2-QZVPP levels of theory.
Characterization of the Simplest Thiolimine – The High Energy Isomer of Thioformamide. Bastian Bernhardt, Friedemann Dressler, André K. Eckhardt, Jonathan Becker, and Peter R. Schreiner
Chem. Eur. J. 2021, 27, 6732–6739. DOI: 10.1002/chem.202005188.
Keep it simple! Thioamides resemble viable prebiotic building blocks for biologically relevant molecules. However, only a handful of experimental reports on parent thioformamide exist. X-ray diffraction data of thioformamide are reported and its tautomerization induced by UV irradiation under cryogenic conditions is presented. Four conformers of the corresponding thiolimine form, whose (tunneling) reactivity is investigated.
As sulfur-containing organic molecules thioamides and their isomers are conceivable intermediates in prebiotic chemistry, for example, in the formation of amino acids and thiazoles and resemble viable candidates for detection in interstellar media. Here, we report the characterization of parent thioformamide in the solid state via single-crystal X-ray diffraction and its photochemical interconversion to its hitherto unreported higher energy tautomer thiolimine in inert argon and dinitrogen matrices. Upon photogeneration, four conformers of thiolimine form, whose ratio depends on the employed wavelength. One of these conformers interconverts due to quantum mechanical tunneling with a half-life of 30–45 min in both matrix materials at 3 and 20 K. A spontaneous reverse reaction from thiolimine to thioformamide is not observed. To support our experimental findings, we explored the potential energy surface of the system at the AE-CCSD(T)/aug-cc-pCVTZ level of theory and computed tunneling half-lives with the CVT/SCT approach applying DFT methods.
Quantum Mechanical Tunneling is Essential to Understanding Chemical Reactivity. Peter R. Schreiner
Trends Chem. 2020, 2, 984–989. DOI: 10.1016/j.trechm.2020.08.006
-
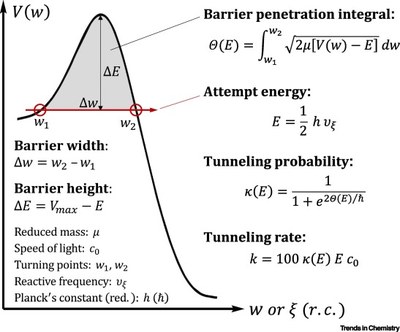
Tunneling is always present but often hidden underneath thermal reactivity. It plays out at low temperatures and can then significantly affect reactivity and selectivity of a chemical reaction.
-
While common for light atoms, in particular, hydrogen, tunneling also occurs for heavier elements when energy barriers are low and narrow, the latter factor being more important.
-
Tunneling control of chemical reactions constitutes, next to kinetic and thermodynamic control, the third paradigm of chemical reactivity.
Competitive nitrogen versus carbon tunnelling. Cláudio M. Nunes, André K. Eckhardt, Igor Reva, Rui Fausto, Peter R. Schreiner
J. Am. Chem. Soc. 2019, 141, 14340–14348. DOI: 10.1021/jacs.9b06869
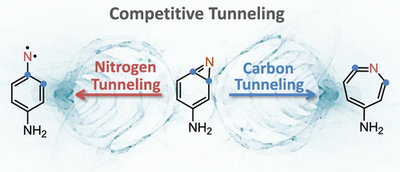
Quantum mechanical tunneling (QMT) of heavy atoms like carbon or nitrogen has been considered very unlikely for the longest time, but recent evidence suggests that heavy-atom QMT does occur more frequently than typically assumed. Here we demonstrate that carbon vs nitrogen heavy-atom QMT can even be competitive leading to two different products originating from the same starting material. Amino-substituted benzazirine was generated in solid argon (3–18 K) and found to decay spontaneously in the dark, with a half-life of 210 min, to p-aminophenylnitrene and amino-substituted ketenimine. The reaction rate is independent of the cryogenic temperature, in contradiction to the rules inferred from classical transition state theory. Quantum chemical computations confirm the existence of two competitive carbon vs nitrogen QMT reaction pathways. This discovery emphasizes the quantum nature of atoms and molecules, thereby enabling a much higher level of control and a deeper understanding of the factors that govern chemical reactivity.
TUNNEX: An easy-to-use Wentzel-Kramer-Brillouin (WKB) implementation to compute tunneling half-lives. Hendrik Quanz and Peter R. Schreiner
J. Comput. Chem. 2019, 41, 543-547. DOI: 10.1002/jcc.25711
Tunneling in experiments (TUNNEX) is a free open‐source program with an easy‐to‐use graphical user interface to simplify the process of Wentzel‐Kramers‐Brillouin (WKB) computations. TUNNEX aims at experimental chemists with basic knowledge of computational chemistry, and it offers the computation of tunneling half‐lives, visualization of data, and exporting of graphs. It also provides a helper tool for executing the zero‐point vibrational energy correction along the path. The program also enables computing high‐level single points along the intrinsic reaction path. TUNNEX is available at https://github.com/prs-group/TUNNEX. As the WKB approximation usually overestimates tunneling half‐lives, it can be used to screen tunneling processes before proceeding with elaborate kinetic experiments or higher‐level tunneling computations such as instanton theory and small curvature tunneling approaches. © 2018 Wiley Periodicals, Inc.
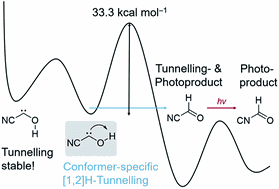
Conformer-Specific [1,2]H-Tunnelling in Captodatively-Stabilized Cyanohydroxycarbene (NC–C̈–OH). André K. Eckhardt, Frederik R. Erb, and Peter R. Schreiner
Chem. Sci. 2019, 9, 802-808. DOI: 10.1039/C8SC03720E
We report the gas-phase preparation of cyanohydroxycarbene by high-vacuum flash pyrolysis of ethyl 2-cyano-2-oxoacetate and subsequent trapping of the pyrolysate in an inert argon matrix at 3 K. After irradiation of the matrix with green light for a few seconds singlet trans-cyanohydroxycarbene rearranges to its cis-conformer. Prolonged irradiation leads to the formation of cyanoformaldehyde and isomeric isocyanoformaldehyde. Cis- and trans-cyanohydroxycarbene were characterized by matching matrix IR and UV/Vis spectroscopic data with ab initio coupled cluster and TD-DFT computations. Trans-cyanohydroxycarbene undergoes a conformer-specific [1,2]H-tunnelling reaction through a 33.3 kcal mol−1 barrier (the highest penetrated barrier of all H-tunnelling reactions observed to date) to cyanoformaldehyde with a half-life of 23.5 ± 0.5 d; this is the longest half-life reported for an H-tunnelling process to date. During the tunnelling reaction the cis-conformer remains unchanged over the same period of time and the Curtin–Hammett principle does not apply. NIR irradiation of the O–H stretching overtone does not enhance the tunnelling rate via vibrational activation. Push–pull stabilisation of hydroxycarbenes through σ- and π-withdrawing groups therefore is even more stabilizing than push–push substitution.
Spectroscopic Evidence for Aminomethylene (H–C̈–NH2) – The Simplest Amino Carbene. André K. Eckhardt and Peter R. Schreiner
Angew. Chem Int. Ed. 2018, 57, 5248–5252. DOI: 10.1002/anie.201800679; Angew. Chem 2018, 130, 5346–5350. DOI: 10.1002/ange.201800679
Highlight: a) Noted as very important paper (top 5% of all Angewandte publications), b) Frontispiece of communications of this issue.

Although N‐heterocyclic carbenes have been well‐studied, the simplest aminocarbene, aminomethylene H−C̈−NH2, has not been spectroscopically identified to date. Herein we report the gas‐phase preparation of aminomethylene by high‐vacuum flash pyrolysis of cyclopropylamine and subsequent trapping of the pyrolysate in an inert argon matrix at 12 K. Aminomethylene was characterized by matching matrix IR and UV/Vis spectroscopic data with ab initio coupled cluster computations. After UV irradiation of the matrix aminomethylene rearranges to its isomer methanimine (formaldimine) H2C=NH. Based on our experimental results and computations aminomethylene has a singlet ground state with a reaction barrier of almost 46 kcal mol−1 to methanimine so that H‐tunneling is excluded.
Intricate Conformational Tunneling in Carbonic Acid Monomethyl Ester. Michael M. Linden, Jan Philipp Wagner, Bastian Bernhardt, Markus A. Bartlett, Wesley D. Allen and Peter R. Schreiner
J. Phys. Chem. Lett. 2018, 9, 1663–1667. DOI: 10.1021/acs.jpclett.8b00295
 Disentangling internal and external effects is a key requirement for understanding conformational tunneling processes. Here we report the s-trans/s-cis tunneling rotamerization of carbonic acid monomethyl ester (1) under matrix isolation conditions and make comparisons to its parent carbonic acid (3). The observed tunneling rate of 1 is temperature-independent in the 3–20 K range and accelerates when using argon instead of neon as the matrix material. The methyl group increases the effective half life (τeff) of the energetically disfavored s-trans-conformer from 3–5 h for 3 to 11–13 h for 1. Methyl group deuteration slows the rotamerization further (τeff ≈ 35 h). CCSD(T)/cc-pVQZ//MP2/aug-cc-pVTZ computations of the tunneling probability suggest that the rate should be almost unaffected by methyl substitution or its deuteration. Thus the observed relative rates are puzzling, and they disagree with previous explanations involving fast vibrational relaxation after the tunneling event facilitated by the alkyl rotor.
Disentangling internal and external effects is a key requirement for understanding conformational tunneling processes. Here we report the s-trans/s-cis tunneling rotamerization of carbonic acid monomethyl ester (1) under matrix isolation conditions and make comparisons to its parent carbonic acid (3). The observed tunneling rate of 1 is temperature-independent in the 3–20 K range and accelerates when using argon instead of neon as the matrix material. The methyl group increases the effective half life (τeff) of the energetically disfavored s-trans-conformer from 3–5 h for 3 to 11–13 h for 1. Methyl group deuteration slows the rotamerization further (τeff ≈ 35 h). CCSD(T)/cc-pVQZ//MP2/aug-cc-pVTZ computations of the tunneling probability suggest that the rate should be almost unaffected by methyl substitution or its deuteration. Thus the observed relative rates are puzzling, and they disagree with previous explanations involving fast vibrational relaxation after the tunneling event facilitated by the alkyl rotor.
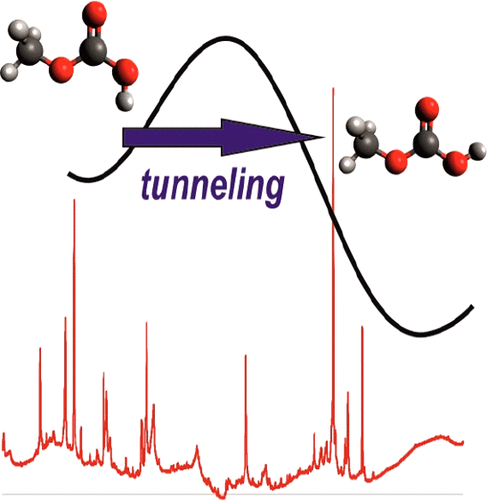
Heavy Atom Secondary Kinetic Isotope Effect on H-Tunneling. André K. Eckhardt, Dennis Gerbig and Peter R. Schreiner
J. Phys. Chem. A 2018, 122, 1488-1495. DOI: 10.1021/acs.jpca.7b12118
Although frequently employed, heavy atom kinetic isotope effects (KIE) have not been reported for quantum mechanical tunneling reactions. Here we examine the secondary KIE through 13C-substitution of the carbene atom in methylhydroxycarbene (H3C–C̈–OH) in its [1,2]H-tunneling 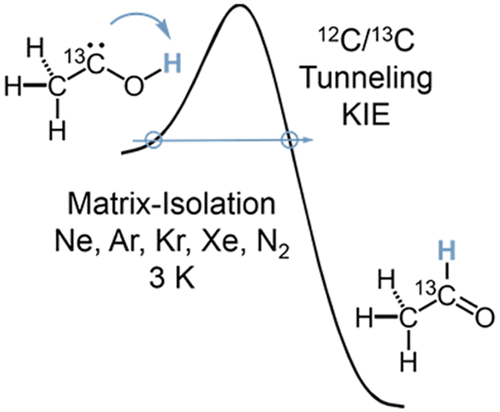 shift reaction to acetaldehyde (H3C–CHO). Our study employs matrix-isolation IR spectroscopy in various inert gases and quantum chemical computations. Depending on the choice of the matrix host gas, the KIE varies within a range of 1.0 in xenon to 1.4 in neon. A KIE of 1.1 was computed using the Wentzel−Kramers−Brillouin (WKB) CVT/SCT, and instanton approaches for the gas phase at the B3LYP/cc-pVTZ level of theory. Computations with explicit consideration of the noble gas environment indicate that the surrounding atoms influence the tunneling reaction barrier height and width. The tunneling half-lives computed with the WKB approach are in good agreement with the experimental results in the different noble gases.
shift reaction to acetaldehyde (H3C–CHO). Our study employs matrix-isolation IR spectroscopy in various inert gases and quantum chemical computations. Depending on the choice of the matrix host gas, the KIE varies within a range of 1.0 in xenon to 1.4 in neon. A KIE of 1.1 was computed using the Wentzel−Kramers−Brillouin (WKB) CVT/SCT, and instanton approaches for the gas phase at the B3LYP/cc-pVTZ level of theory. Computations with explicit consideration of the noble gas environment indicate that the surrounding atoms influence the tunneling reaction barrier height and width. The tunneling half-lives computed with the WKB approach are in good agreement with the experimental results in the different noble gases.
Tunneling Control of Chemical Reactions: The Third Reactivity Paradigm. (Invited Perspective) Peter R. Schreiner J. Am. Chem. Soc. 2017, 139, 15276–15283. DOI: 10.1021/jacs.7b06035. On the list of “most read articles” in 2017. Corrigendum: J. Am. Chem. Soc. 2018, 140, 1566. DOI: 10.1021/jacs.8b00446
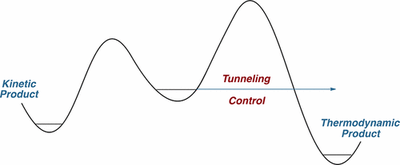
This Perspective describes the emergence of tunneling control as a new reactivity paradigm in chemistry. The term denotes a tunneling reaction that passes through a high but narrow potential energy barrier, leading to formation of a product that would be disfavored if the reaction proceeded by passage over kinetic barriers rather than through them. This reactivity paradigm should be considered along with thermodynamic and kinetic control as a factor that can determine which of two or more possible products is likely to be the one obtained. Tunneling control is a concept that can provide a deep and detailed understanding of a variety of reactions that undergo facile (and possibly unrecognized) tunneling.
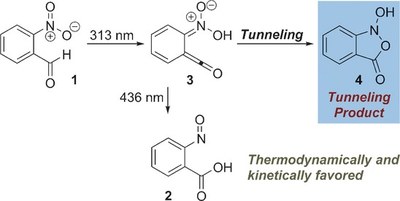
Formation of a Tunneling Product in the Photo-Rearrangement of o-Nitrobenzaldehyde.
Dennis Gerbig and Peter R. Schreiner Angew. Chem. Int. Ed. 2017, 56, in 9445-9448. DOI: 10.1002/anie.201705140
The photochemical rearrangement of o-nitrobenzaldehyde to o-nitrosobenzoic acid, first reported in 1901, has been shown to proceed via a distinct ketene intermediate. In the course of matrix isolation experiments in various host materials at temperatures as low as 3 K, the ketene was re-investigated in its electronic and vibrational ground states. It was shown that hitherto unreported H-tunneling dominates its reactivity, with half-lives of a few minutes. Unexpectedly, the tunneling product is different from o-nitrosobenzoic acid formed in the photoprocess: Once prepared by irradiation, the ketene spontaneously rearranges to an isoxazolone via an intriguing mechanism initiated by H-tunneling. CCSD(T)/cc-pVTZ computations reveal that this isoxazolone is neither thermodynamically nor kinetically favored under the experimental conditions, and that formation of this unique tunneling product constitutes a remarkable and new example of tunneling control.
Isotope-Controlled Selectivity by Quantum Tunneling: Hydrogen Migration versus Ring Expansion in Cyclopropylmethylcarbenes. Ashim Nandi, Dennis Gerbig, Peter R. Schreiner Weston T. Borden, and Sebastian Kozuch J. Am. Chem. Soc. 2017, 139, 9097–9099. DOI: 10.1021/jacs.7b04593
Using the tunneling-controlled reactivity of cyclopropylmethylcarbene, we demonstrate the 
Trifluoromethylhydroxycarbene: Conformer-specific hydrogen-atom tunneling. Artur Mardyukov, Henrik Quanz, and Peter R. Schreiner*
Nature Chem. 2017, 9, 71–77. DOI: 10.1038/nchem.2609
Conformational control of organic reactions is at the heart of the biomolecular sciences. To 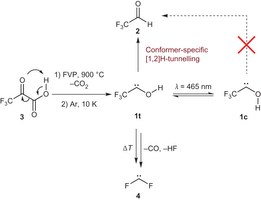
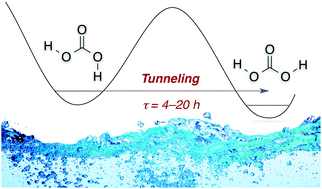
Tunneling in Carbonic Acid. J. Philipp Wagner, Hans-Peter Reisenauer, Vivii Hirvonen, Chia-Hua Wu, Joseph L. Tyberg, Wesley D. Allen and Peter R. Schreiner
Chem. Commun. 2016, 52, 7858–7861. DOI: 10.1039/c6cc01756h
The cis,trans-conformer of carbonic acid (H2CO3), generated by near-infrared radiation, undergoes an unreported quantum mechanical tunnelling rotamerization with half-lives in cryogenic matrices of 4–20 h, depending on temperature and host material. First-principles quantum chemistry at high levels of theory gives a tunnelling half-life of about 1 h, quite near those measured for the fastest rotamerizations.
Domino Tunneling. Peter R. Schreiner,* J. Philipp Wagner, H. P. Reisenauer, Dennis Gerbig, David Ley, János Sarka, Attila G. Császár, Alexander Vaughn and Wesley D. Allen
J. Am. Chem. Soc. 2015, 137, 7828–7834. DOI: 10.1021/jacs.5b03322
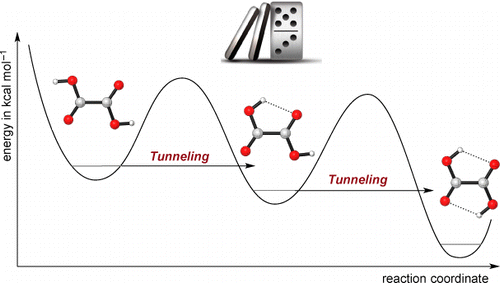
Matrix-isolation experiments near 3 K and state-of-the-art quantum chemical computations demonstrate that oxalic acid [1, (COOH)2] exhibits a sequential quantum mechanical tunneling phenomenon not previously observed. Intensities of numerous infrared (IR) bands were used to monitor the temporal evolution of the lowest-energy O–H rotamers (1cTc, 1cTt, 1tTt) of oxalic acid for up to 19 days following near-infrared irradiation of the matrix. The relative energies of these rotamers are 0.0 (1cTc), 2.6 (1cTt), and 4.0 (1tTt) kcal mol–1. A 1tTt → 1cTt → 1cTc isomerization cascade was observed with half-lives (t1/2) in different matrix sites ranging from 30 to 360 h, even though the sequential barriers of 9.7 and 10.4 kcal mol–1 are much too high to be surmounted thermally under cryogenic conditions. A general mathematical model was developed for the complex kinetics of a reaction cascade with species in distinct matrix sites. With this model, a precise, global nonlinear least-squares fit was achieved simultaneously on the temporal profiles of nine IR bands of the 1cTc, 1cTt, and 1tTt rotamers. Classes of both fast (t1/2 = 30–50 h) and slow (t1/2 > 250 h) matrix sites were revealed, with the decay rate of the former in close agreement with first-principles computations for the conformational tunneling rates of the corresponding isolated molecules. Rigorous kinetic and theoretical analyses thus show that a “domino” tunneling mechanism is at work in these oxalic acid transformations.
Hydrogen-Tunneling in Biologically Relevant Small Molecules: The Rotamerization of a-Ketocarboxyxlic Acids. Dennis Gerbig and Peter R. Schreiner J. Phys. Chem. B 2015, 119, 693–703. DOI: 10.1021/jp503633m
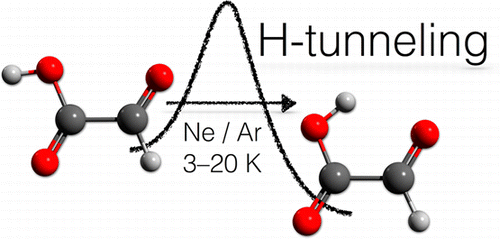 Quantum mechanical tunneling governs the C–O bond rotamerization of simple alkyl and aryl carboxylic acid conformers at cryogenic temperatures. In this study, we report tunneling investigations on a series of electronically different α-ketocarboxylic acids including glyoxylic, pyruvic, cyclopropylglyoxylic, and phenylglyoxylic acid in solid Ar and Ne as host materials at temperatures ranging from 3 to 20 K. The higher-lying rotamers generated through photoirradiation with wavelengths of λ = 313 nm or λ > 850 nm convert to their low-energy conformers through hydrogen-tunneling, as evident from the time evolution of their infrared spectra, and the complete suppression of this process by deuteration. The conversion rates sensitively depend on the choice of matrix material and the tunneling half-lives range from a few hours to several days and are higher in Ne than in Ar for glyoxylic, pyruvic, and cyclopropylglyoxylic acid. The advent of tunneling in α-ketocarboxylic acids dominates their conformational preferences and conceivably also the reactivity of biologically and pharmacologically relevant acid congeners.
Quantum mechanical tunneling governs the C–O bond rotamerization of simple alkyl and aryl carboxylic acid conformers at cryogenic temperatures. In this study, we report tunneling investigations on a series of electronically different α-ketocarboxylic acids including glyoxylic, pyruvic, cyclopropylglyoxylic, and phenylglyoxylic acid in solid Ar and Ne as host materials at temperatures ranging from 3 to 20 K. The higher-lying rotamers generated through photoirradiation with wavelengths of λ = 313 nm or λ > 850 nm convert to their low-energy conformers through hydrogen-tunneling, as evident from the time evolution of their infrared spectra, and the complete suppression of this process by deuteration. The conversion rates sensitively depend on the choice of matrix material and the tunneling half-lives range from a few hours to several days and are higher in Ne than in Ar for glyoxylic, pyruvic, and cyclopropylglyoxylic acid. The advent of tunneling in α-ketocarboxylic acids dominates their conformational preferences and conceivably also the reactivity of biologically and pharmacologically relevant acid congeners.
Tunneling Control of Chemical Reactions: C–H Insertion versus H-Tunneling in tert-Butylhydroxycarbene. David Ley, Dennis Gerbig, and Peter R. Schreiner Chem. Sci. 2013, 4, 677–684. DOI: 10.1039/C2SC21555A.
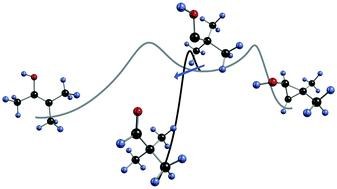 Elusive tert-butylhydroxycarbene was generated in the gas phase via high-vacuum flash pyrolysis of tert-butylglyoxylic acid at 960 °C. The pyrolysis products were subsequently matrix isolated in solid Ar at 11 K and characterized by means of IR spectroscopy. While still being exposed to the harsh pyrolysis conditions, the hydroxycarbene undergoes CH-insertion to dimethylcyclopropanol, as well as a CC-insertion to novel methylbutenol, with activation barriers of 23.8 and 31.0 kcal mol−1, respectively. Once embedded in the cold Ar matrix, the carbene transforms to its isomer pivaldehyde not only by photolysis, but it also cuts through the barrier of 27.3 kcal mol−1 by quantum mechanical tunneling. The temperature independent half-life is measured as 1.7 h; the tunneling pathway was entirely blocked upon O-deuteration. The experimental half-life of tert-butylhydroxycarbene was verified by tunneling computations applying the Wentzel–Kramers–Brillouin formalism on the minimum energy path evaluated at the computationally feasible M06-2X/6-311++G(d,p) level of theory. Our experimental findings are supported by relative energy computations at the CCSD(T)/cc-pVDZ level of theory.
Elusive tert-butylhydroxycarbene was generated in the gas phase via high-vacuum flash pyrolysis of tert-butylglyoxylic acid at 960 °C. The pyrolysis products were subsequently matrix isolated in solid Ar at 11 K and characterized by means of IR spectroscopy. While still being exposed to the harsh pyrolysis conditions, the hydroxycarbene undergoes CH-insertion to dimethylcyclopropanol, as well as a CC-insertion to novel methylbutenol, with activation barriers of 23.8 and 31.0 kcal mol−1, respectively. Once embedded in the cold Ar matrix, the carbene transforms to its isomer pivaldehyde not only by photolysis, but it also cuts through the barrier of 27.3 kcal mol−1 by quantum mechanical tunneling. The temperature independent half-life is measured as 1.7 h; the tunneling pathway was entirely blocked upon O-deuteration. The experimental half-life of tert-butylhydroxycarbene was verified by tunneling computations applying the Wentzel–Kramers–Brillouin formalism on the minimum energy path evaluated at the computationally feasible M06-2X/6-311++G(d,p) level of theory. Our experimental findings are supported by relative energy computations at the CCSD(T)/cc-pVDZ level of theory.
Tunneling Control of Chemical Reactions – The Organic Chemist’s Perspective. David Ley, Dennis Gerbig, and Peter R. Schreiner*
Org. Biomol. Chem. 2012, 10, 3781–3790. DOI: 10.1039/c2ob07170c. 
Highlight: Front cover of this issue. Listed as one of the 10 most accessed
articles [http://blogs.rsc.org/ob/category/top-10/].
Even though quantum mechanical tunnelling has been appearing recurrently mostly in theoretical studies that emphasize its decisive role for many chemical reactions, it still appears suspicious to most organic chemists. Recent experiments in combination with powerful computational approaches, however, have demonstrated that tunnelling must be included to fully understand chemical reactivity. Here we provide an overview of the importance of tunnelling in organic chemical reactions.
Cyclopropylhydroxycarbene. David Ley, Dennis Gerbig, J. Philipp Wagner, Hans Peter Reisenauer, and Peter R. Schreiner* J. Am. Chem. Soc. 2011, 133, 13614–13621. Download
Cyclopropylhydroxycarbene was generated by high-vacuum flash pyrolysis of cyclopropylglyoxylic acid at 960 °C. The pyrolysis products were matrix-isolated in solid Ar at 11 K and characterized by means of IR spectroscopy. Upon photolysis, the carbene undergoes ring expansion, thereby paralleling the reactivity of other known cyclopropylcarbenes. The ring expansion product, cyclobut-1-en-1-ol, was characterized for the first time. Matrix-isolated cyclopropylhydroxycarbene undergoes [1,2]H-tunneling through a barrier of approximately 30 kcal·mol–1, yielding cyclopropylcarboxaldehyde. The cyclopropyl moiety acts as a π-donor and increases the half-life by almost a factor of 10 compared to parent hydroxymethylene, resulting in a temperature-independent half-life of τ = 17.8 h at both 11 and 20 K. Hence, cyclopropylhydroxycarbene is the first hydroxycarbene that differs from other members of its family by a significantly prolonged half-life. As expected, the O-deuterated analogue does not show tunneling. Our findings are rationalized by accurate CCSD(T)/cc-pVnZ (n = D, T)//M06-2X/6-311++G(d,p) computations. The half-life of cyclopropylhydroxycarbene was verified by tunneling computations employing the Wentzel–Kramers–Brillouin formalism. By comparison with other experimentally known hydroxycarbenes, we determine the electronic donor capabilities of the carbenes’ substituents to be a dominant factor governing their half-lives.
Methylhydroxycarbene: Tunneling Control of a Chemical reaction. Peter R. Schreiner, Hans Peter Reisenauer, David Ley, Dennis Gerbig, Chia-Hua Wu, and Wesley D. Allen Science 2011, 332, 1300–1303. Download Perspective: Taking the high road and getting there before you. Barry K. Carpenter Science 2011, 332, 1269–1270. Highlights: a) Quantum tunneling creates “wrong” molecule. Laura Howes Chemistry World 2011, June 10. b) Tunneleffekt wandelt Methylhydroxycarben in Acetaldehyd um. Reto Müller Organic Chemistry Portal 2011, June 14. c) Verblüffender chemischer Tunnelverkehr Scienceticker 2011, June 9. d) Das Beamen von Materie: Neue Triebkraft chemischer Reaktionen entdeckt Chemie.de 2011, June 16.
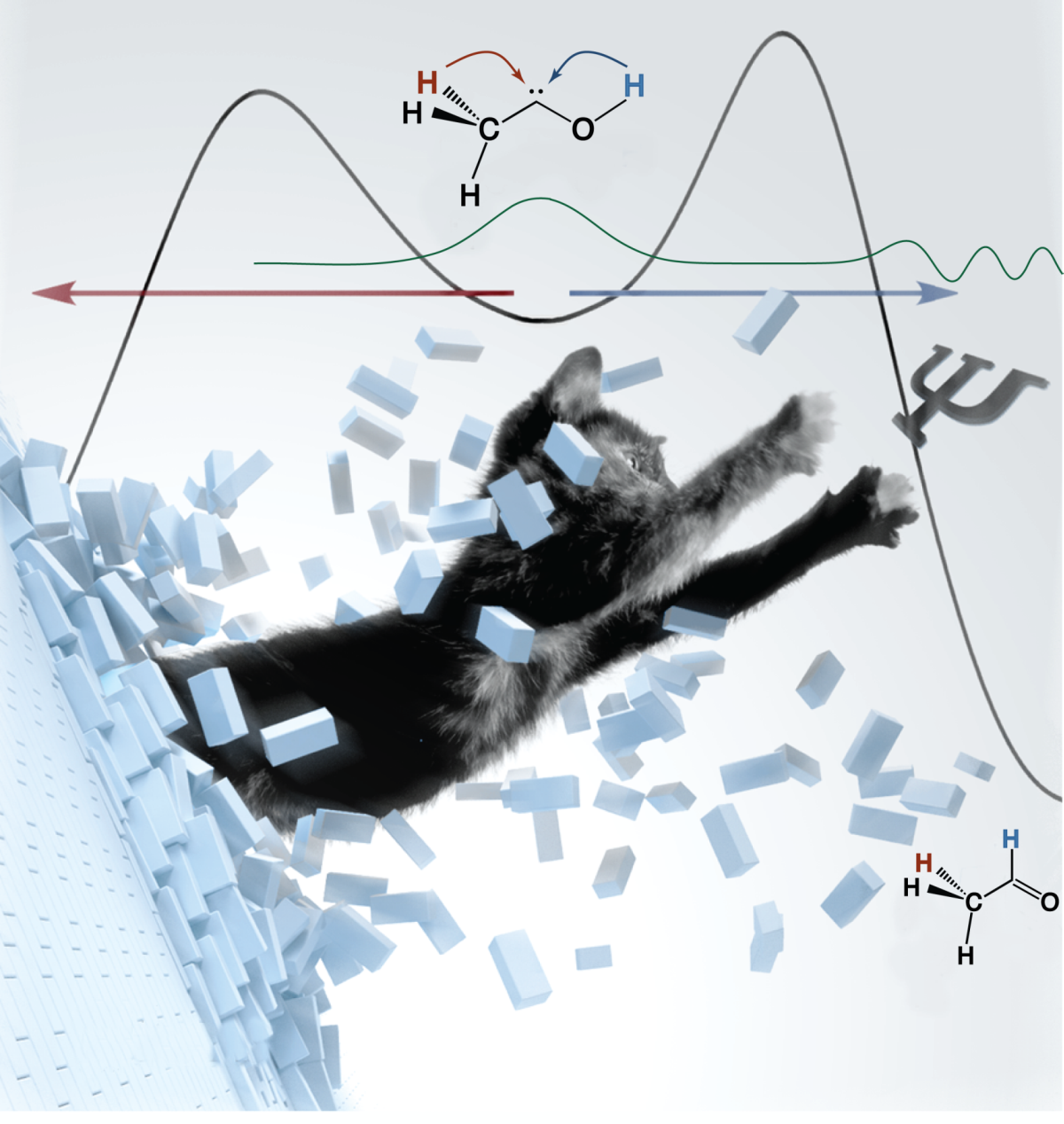 Chemical reactivity is conventionally understood in broad terms of kinetic versus thermodynamic control, wherein the decisive factor is the lowest activation barrier among the various reaction paths or the lowest free energy of the final products, respectively. Here we demonstrate that quantum mechanical tunneling can supersede traditional kinetic control and direct a reaction exclusively to a product whose reaction path has a higher barrier. Specifically, we prepared methylhydroxycarbene (H3C–C–OH) via vacuum pyrolysis of pyruvic acid at ca. 1200 Kelvin (K), followed by argon matrix trapping at 11 K. The previously elusive carbene, characterized by ultraviolet (UV) and infrared (IR) spectroscopy as well as exacting quantum mechanical computations, undergoes a facile [1,2]hydrogen shift to acetaldehyde via tunneling under a barrier of 28.0 kilocalories per mole (kcal mol–1) with a half-life around 1 hour (h). The analogous isomerization to vinyl alcohol has a substantially lower barrier of 22.6 kcal mol–1 but is precluded at low temperature by the greater width of the potential energy profile for tunneling.
Chemical reactivity is conventionally understood in broad terms of kinetic versus thermodynamic control, wherein the decisive factor is the lowest activation barrier among the various reaction paths or the lowest free energy of the final products, respectively. Here we demonstrate that quantum mechanical tunneling can supersede traditional kinetic control and direct a reaction exclusively to a product whose reaction path has a higher barrier. Specifically, we prepared methylhydroxycarbene (H3C–C–OH) via vacuum pyrolysis of pyruvic acid at ca. 1200 Kelvin (K), followed by argon matrix trapping at 11 K. The previously elusive carbene, characterized by ultraviolet (UV) and infrared (IR) spectroscopy as well as exacting quantum mechanical computations, undergoes a facile [1,2]hydrogen shift to acetaldehyde via tunneling under a barrier of 28.0 kilocalories per mole (kcal mol–1) with a half-life around 1 hour (h). The analogous isomerization to vinyl alcohol has a substantially lower barrier of 22.6 kcal mol–1 but is precluded at low temperature by the greater width of the potential energy profile for tunneling.
Tunneling in trans-HCSH and trans-HCSeH. János Sarka, Attila G. Császár, and Peter R. Schreiner Collect. Czech. Chem. Commun. 2011, 76, 645–667. Download The principal purpose of this investigation is the determination of the tunneling half-lives of the trans-HCSH → H2CS and the trans-HCSeH → H2CSe unimolecular isomerization reactions at temperatures close to 0 K. To aid these determinations, accurate electronic structure computations were performed, with electron correlation treatments as extensive as CCSDT(Q) and basis sets as large as aug-cc-pCV5Z, for the isomers of [H,H,C,S] and [H,H,C,Se] on their lowest singlet surfaces and for the appropriate transition states yielding structural data for key stationary points characterizing the isomerization reactions. The computational results were subjected to a focal-point analysis (FPA) that yields accurate relative energies with uncertainty estimates. The tunneling half-lives were determined by a simple Eckart-barrier approach and via the more sophisticated though still one-dimensional Wentzel–Kramers–Brillouin (WKB) approximation. Only stationary-point information is needed for the former while an intrinsic reaction path (IRP) is necessary for the latter approach. Both protocols suggest that, unlike for the parent hydroxymethylene (HCOH), at the low temperatures of matrix isolation experiments no tunneling will be observable for the trans-HCSH and trans-HCSeH systems.
The principal purpose of this investigation is the determination of the tunneling half-lives of the trans-HCSH → H2CS and the trans-HCSeH → H2CSe unimolecular isomerization reactions at temperatures close to 0 K. To aid these determinations, accurate electronic structure computations were performed, with electron correlation treatments as extensive as CCSDT(Q) and basis sets as large as aug-cc-pCV5Z, for the isomers of [H,H,C,S] and [H,H,C,Se] on their lowest singlet surfaces and for the appropriate transition states yielding structural data for key stationary points characterizing the isomerization reactions. The computational results were subjected to a focal-point analysis (FPA) that yields accurate relative energies with uncertainty estimates. The tunneling half-lives were determined by a simple Eckart-barrier approach and via the more sophisticated though still one-dimensional Wentzel–Kramers–Brillouin (WKB) approximation. Only stationary-point information is needed for the former while an intrinsic reaction path (IRP) is necessary for the latter approach. Both protocols suggest that, unlike for the parent hydroxymethylene (HCOH), at the low temperatures of matrix isolation experiments no tunneling will be observable for the trans-HCSH and trans-HCSeH systems.
Light- and heavy atom tunneling in rearrangement reactions of cyclopropylcarbenes. Dennis Gerbig, David Ley, and Peter R. Schreiner* Org. Lett. 2011, 13, 3526–3529. Download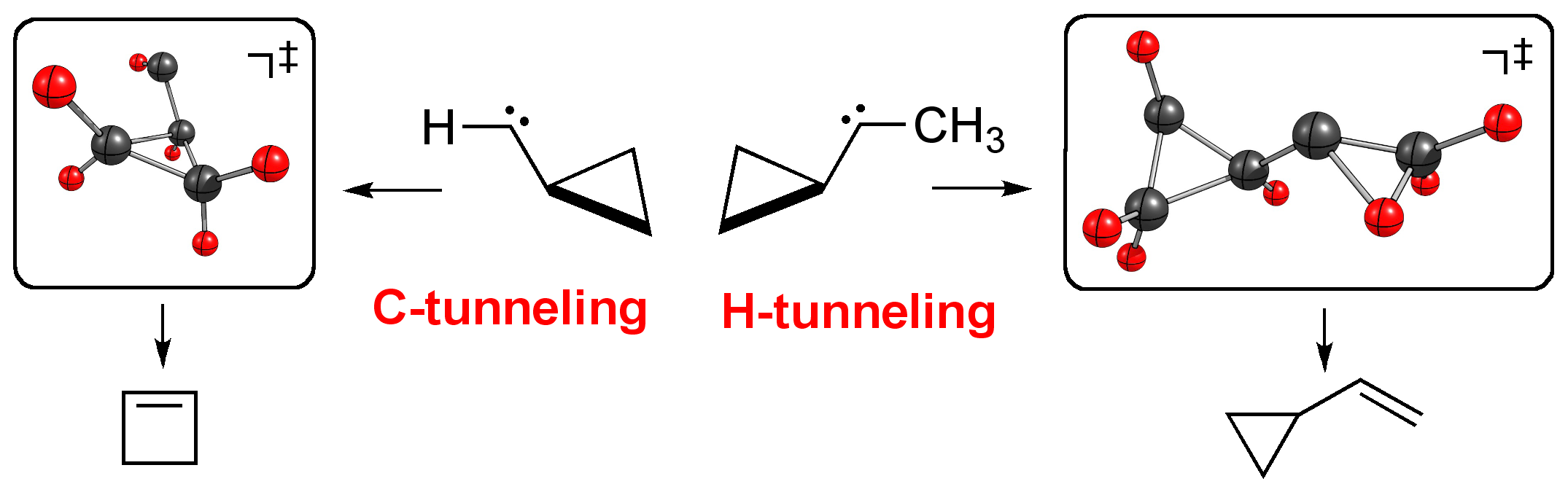
We investigated both light- and heavy-atom tunneling in the rearrangements of a series of cyclopropylcarbenes using canonical variational transition state theory with multidimensional tunneling corrections (CVT/MT) and the Wentzel-Kramers-Brillouin (WKB) formalism. Halogeno- and hydroxy-substituted cyclopropylcarbenes were found not to undergo carbon tunneling owing to wide reaction barriers. However, while carbon tunneling plays a major role in the ring expansion of parent cyclopropylcarbene yielding cyclobutene, cyclopropylmethylcarbene is prone to undergo hydrogen tunneling to give cyclopropylmethylene.
Electronic Effects on Atom Tunneling: Conformational Isomerization of Monomeric para-Substituted Benzoic Acids Derivatives. Shadi Amiri, Hans Peter Reisenauer, and Peter R. Schreiner J. Am. Chem. Soc. 2010, 132, 15902–15904. Download
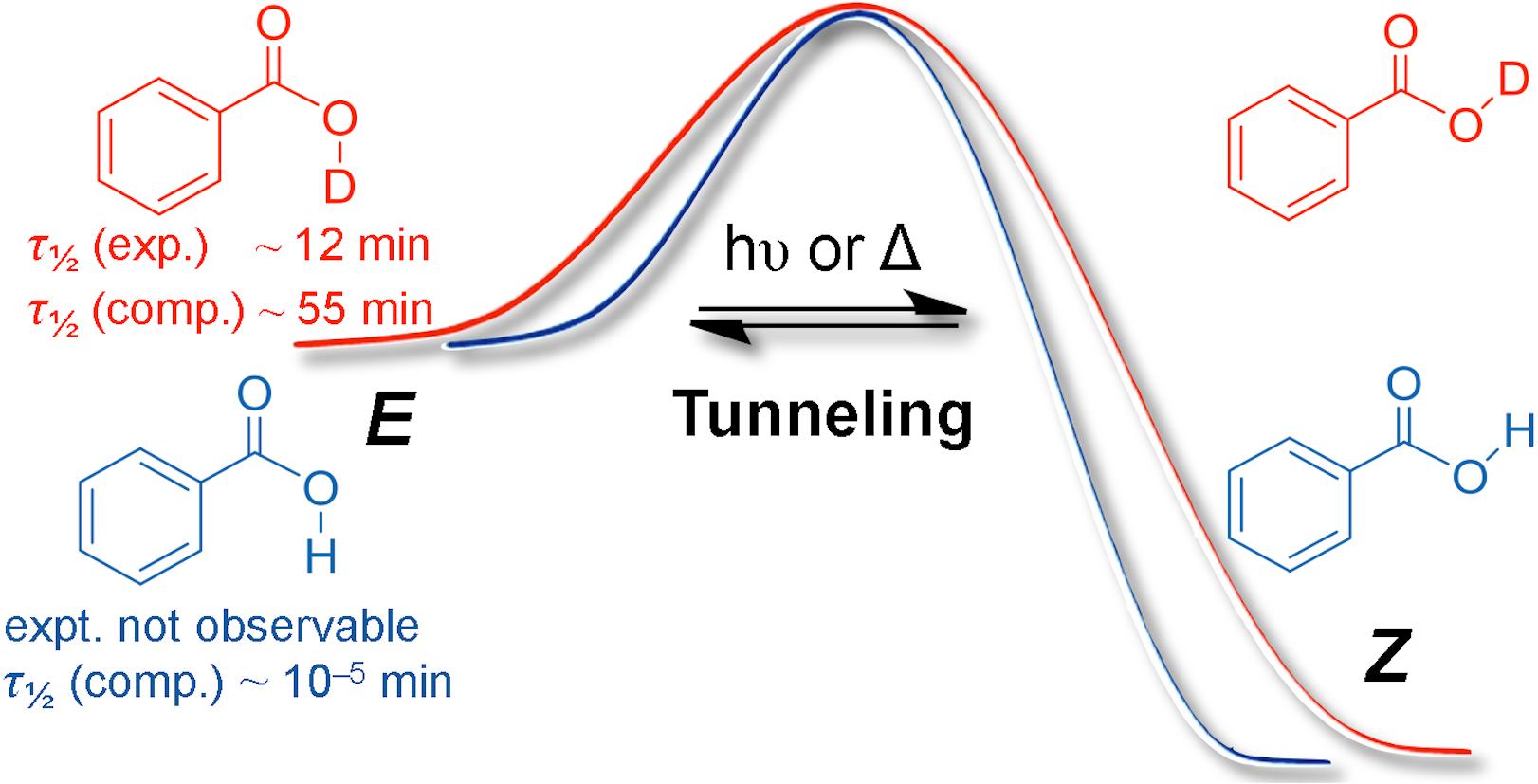
We present the first generation and spectroscopic identification of the higher-lying E-conformer of the simplest aromatic carboxylic acid, benzoic acid (1a) as its O-deuterated isotopologue (E-d1-1a) using matrix isolation techniques; parent E-1a could not be observed owing to fast H-tunneling to more stable Z-1a. Even deuterated E-d1-1a converts quickly back to Z-d1-1a through D-tunneling with a half-life (τ) of ca. 12 min in Ar at 11 K. Tunneling computations using an Eckart barrier in conjunction with a CCSD(T)/cc-pVTZ//MP2/cc-pVDZ + ZPVE intrinsic reaction path reveal that τ of E-1a is only ca. 10–5 min, in marked contrast to simple aliphatic acids that have half-lives in the range of minutes. The electronic substituent effects on D-tunneling in para-substituted benzoic acid derivatives (p-X-PhCOOD, d1-1) were systematically studied in Ar matrices at 11 K. σ-Electron donors (X = alkyl) increase the half lives of d1-1, while σ-acceptor/π-donor groups (X = OD, NH2, Hal) and even more so σ/π-acceptors (X = NO2) decrease τ. The latter finding is in line with the smaller E-to-Z-reaction barriers and narrower reaction widths for the isomerization. Tunneling substituent constants (σt) for this conformational isomerization were derived experimentally and computationally.
Intramolecular Hydroxycarbene C–H Insertions: The Curious Case of (o-Methoxy)phenylhydroxycarbene. Dennig Gerbig, David Ley, Hans Peter Reisenauer, Peter R. Schreiner* Beilstein J. Org. Chem. 2010, 6, 1061–1069. Download
Phenylhydroxycarbene. Dennis Gerbig, Hans Peter Reisenauer, Chia-Hua Wu, David Ley, Wesley D. Allen, Peter R. Schreiner J. Am. Chem. Soc. 2010, 132, 7273–7275. Download
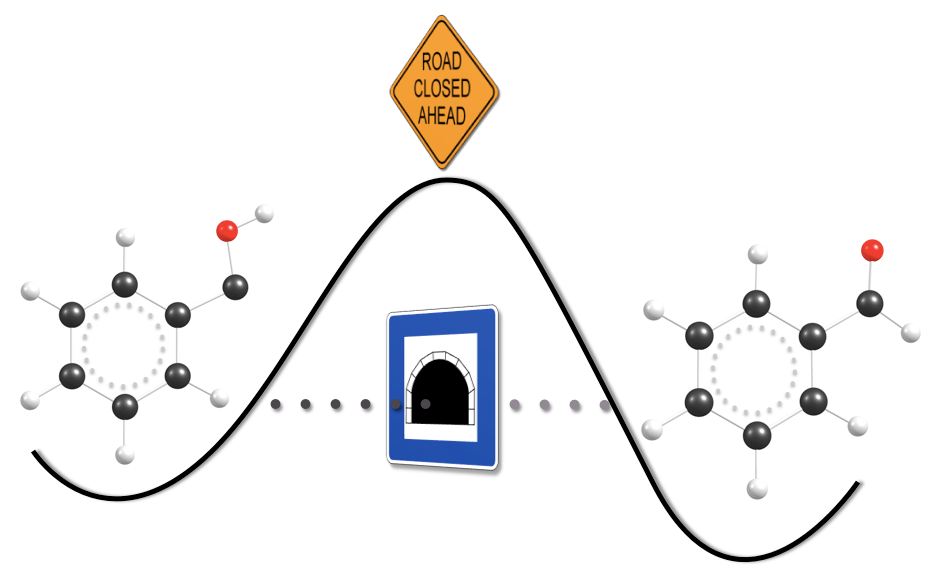
Phenylhydroxycarbene (1, Ph–C–OH), the parent of all arylhydroxycarbenes, was generated by high vacuum flash pyrolysis of phenylglyoxylic acid (at 600 °C) and spectroscopically (IR, UV/Vis) characterized via immediate matrix isolation in solid Ar at 11 K. The identity of 1 was unequivocally confirmed by precise agreement between observed IR bands and (unscaled) anharmonic vibrational frequencies computed from a CCSD(T)/cc-pVDZ quartic force field. The UV/Vis spectrum of 1 displays a broad band with maximum absorption at 500 ± 25 nm (2.5 ± 0.1 eV) that extends to around 640 nm (1.9 eV), in full accord with combined CCSD(T)/cc-pVQZ and EOM-CCSD/cc-pVTZ computations that yield a gas-phase vertical (adiabatic) excitation energy of 2.7 (1.9) eV. Unlike singlet phenylchlorocarbene, 1 does not undergo photochemical ring expansion. Instead, 1 exhibits quantum mechanical hydrogen tunneling to benzaldehyde underneath a formidable barrier of 28.8 kcal mol–1, even at cryogenic temperatures. The remarkable hydrogen tunneling mechanism is supported by the temperature insensitivity of the observed half-life (2.5 h) and is substantiated by a comparable theoretical half-life (3.3 h) determined from high-level barrier penetration integrals computed along the intrinsic reaction path. As expected, deuteration turns off the tunneling mechanism, so that d-1 is stable under otherwise identical conditions.
Spectroscopic Characterization of Dihydroxycarbene. Peter R. Schreiner and Hans Peter Reisenauer Angew. Chem. Int. Ed. 2008, 47, 7071. Angew. Chem. 2008, 120, 7179. Download
Abstract. CO2 extrusion from simple α-keto carboxylic acids proofs to be a novel method of general utility for the preparation of hitherto unknown hydroxycarbenes. Dihydroxycarbene as well as methoxyhydroxycarbene, which are both stabilized through π-type out-of-plane as well as in-plane σ-conjugation, can thus be prepared and fully characterized. |
 |
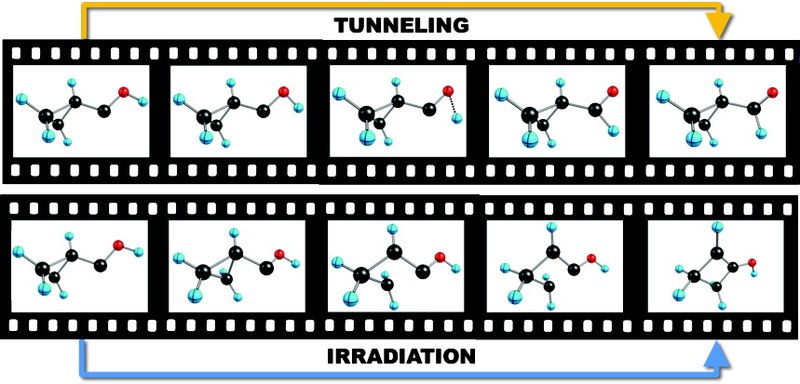
Capture of Elusive Hydroxymethylene, a Tunnelling Marvel Precluded from Sugar Formation in Space. P. R. Schreiner, H. P. Reisenauer, F. Pickard, A. C. Simmonett, W. D. Allen, E. Mátyus, and A. G. Császár Nature 2008, 453, 906. Highlights: a) Cool it, baby. M. Räsanen Nature 2008, 453, 862–863. b) Houdini molecule escapes energy trap. S. Hadlington Chemistry World 2008, June 11, p. 23. c) Hydroxymethylene Captured. B. Halford Chem. Eng. News 2008, 86(24), 15. d) Leben eines Organischen Moleküls verlängert. U. Bilow FAZ, August 6, 2008, 182, p. N1. e) Watching a Molecular Mole at Work. G. Bucher Angew. Chem. Int. Ed. 2008, 47, 6957–6958. f) Aus der Traum vom Weltraum? S. Feil Chem. Unserer Zeit 2008, 42, 252. Download
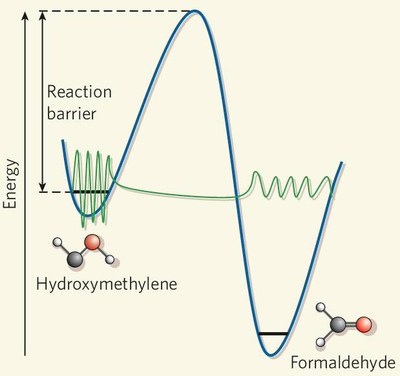
Abstract. Singlet Carbenes exhibit a divalent carbon atom whose valence shell contains only six electrons, four involved in bonding to two other atoms and the remaining two forming a non-bonding electron pair. These features render singelt carbenes so reactive that they were long considered too short-lived for isolation and direct characterization. This view changed when it was found that attaching the divalent carbon atom to substituents that are bulky and/or able to donate electrons produces carbenes that can be isolated and stored. N-heterocyclic carbenes are such compounds now in wide use, for example as ligands in metathesis catalysis. In contrast, oxygen-donor-substituted carbenes are inherently less stable and have been less studied. The pre-eminent case is hydroxymethylene, H–C–OH although it is the key intermediate in the high-energy chemistry of its tautomer formaldehyde, has been implicated since 1921 in the photocatalytic formation of carbohydrates, and is the parent of alkoxycarbenes that lie at the heart of transition-metal carbene chemistry, all attempts to observe this species or other alkoxycarbenes have failed. However, theoretical considerations indicate that hydroxymethylene sould be isolatable. Here we report the synthesis of hydroxymethylene and its capture by matrix isolation. We unexpectedly find that H–C–OH rearranges to formaldehyde with a half-life of only 2 h at 11 K by pure hydrogen tunneling through a large energy barrier in excess of 30 kcal mol–1.
