Reactive Intermediates
Singlet carbenes incorporating a divalent carbon atom (R–C–R’) have grown from laboratory curiosities and theoreticians’ pet peeves into reagents in the growing field of stable (i.e., heterocyclic) carbene chemistry. Still, the experimental characterization of many other simple yet fundamentally important reactive species such as alkyl- or hydroxycarbenes, silylenes or sulfenes is hampered by their high reactivity or lack of precursors: Hydroxycarbenes, for instance, have been an unknown class of compounds until 2008, when our group reported the synthesis and characterization of hydroxymethylene (H–C–OH), whose preparation has been challenging organic chemists for more than 80 years. The reaction of hydroxycarbene with formaldehyde would be a source of simple sugars (the so-called “formose reaction” in the origin of life theory). Considerable efforts are ongoing to understand the formation and distribution of a wide variety of simple organics in extraterrestrial environments, and the examination of the structures and reactivities of prototypes such as various hydroxycarbenes, simple carboxylic acids or seemingly "exotic" heteroatom-containing compounds may also provide glimpses of the prebiotic earth.
Generation and Spectroscopic Identification of the Thiuram Radical (CH3)2NCS2∙
Artur Mardyukov, Felix Keul, and Peter R. Schreiner
J. Phys. Chem. A 2019, 123, 4937–4941. DOI: 10.1021/acs.jpca.9b03307
We report the first preparation, matrix-isolation, and IR and UV/vis spectroscopic characterization of the thiuram radical that is a highly important species for many industrial processes. The thiuram radical was prepared by thermal dissociation of tetramethylthiuram disulfide and was identified by matching its spectroscopic data with density functional theory [UB3LYP/6-311++G(3df,3pd)] computations. The title compound proved to be highly photolabile, and irradiation with light at λ = 623 nm affords a hitherto unknown carbamodithioic acid, N-(methyl)-N-methyl radical, as characterized by IR and UV/vis spectroscopy in low-temperature matrices.
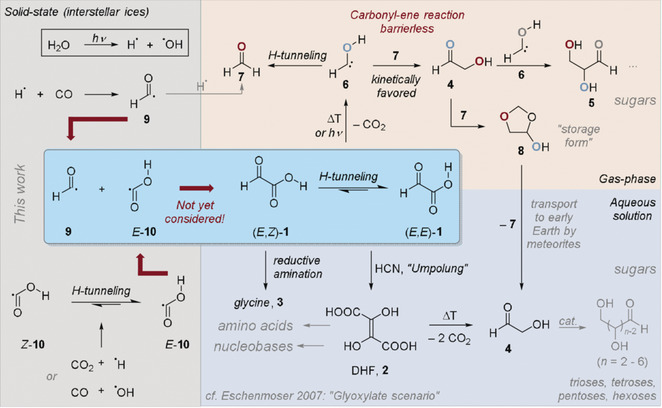
Formation of Glyoxylic Acid (HCOCOOH) in Interstellar Ices – A Key Entry Point for Prebiotic Chemistry
André K. Eckhardt, Alexandre Bergantini, Santosh K. Sing, Peter R. Schreiner and Ralf I. Kaiser
Angew. Chem. Int. Ed. 2019, 58, 5663–5667. DOI: 10.1002/anie.201901059
With nearly 200 molecules detected in interstellar and circumstellar environments, the identification of the biologically relevant α‐keto carboxylic acid, glyoxylic acid (HCOCOOH), is still elusive. Herein, the formation of glyoxylic acid via cosmic‐ray driven, non‐equilibrium chemistry in polar interstellar ices of carbon monoxide (CO) and water (H2O) at 5 K via barrierless recombination of formyl (HCO) and hydroxycarbonyl radicals (HOCO) is reported. In temperature‐programmed desorption experiments, the subliming neutral molecules were selectively photoionized and identified based on the ionization energy and distinct mass‐to‐charge ratios in combination with isotopically labeled experiments exploiting reflectron time‐of‐flight mass spectrometry. These studies unravel a key reaction path to glyoxylic acid, an organic molecule formed in interstellar ices before subliming in star‐forming regions like SgrB2(N), thus providing a critical entry point to prebiotic organic synthesis.
Caged Nitric Oxide-Thiyl Radical Pairs
Zhuang Wu, Changyun Chen, Jie Liu, Yan Lu, Jian Xu, Jian, Xiangya Liu, Ganglong Cui, Tarek Trabelsi, Joseph Francisco, Artur Mardyukov, André K. Eckhardt, Peter R. Schreiner, Xiaoqing Zeng
J. Am. Chem. Soc. 2019, 141, 3361–3365. DOI: 10.1021/jacs.8b12746
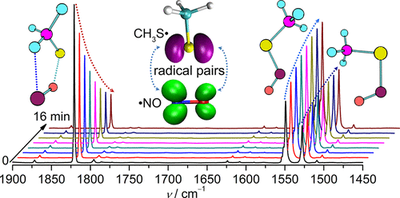
S-Nitrosothiols (RSNO) are exogenous and endogenous sources of nitric oxide in biological systems due to facile homolytic cleavage of the S–N bonds. By following the photolytic decomposition of prototypical RSNO (R = Me and Et) in Ne, Ar, and N2 matrixes (<10 K), elusive caged radical pairs consisting of nitric oxide (NO•) and thiyl radicals (RS•), bridged by O···S and H···N connections, were identified with IR and UV/vis spectroscopy. Upon red-light irradiation, both caged radical pairs (RS•···•ON) vanish and reform RSNO. According to the calculation at the CASPT2(10,8)/cc-pVDZ level (298.15 K), the dissociation energy of MeS•···•ON amounts to 4.7 kcal mol–1.
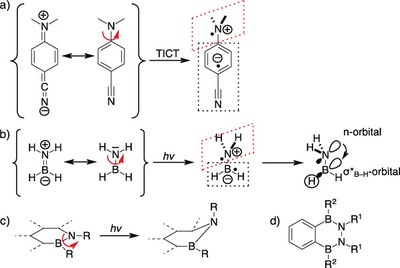
Control of excited state conformations of B,N-heterocycles
Zhenpin Lu, Julia Ruhl, Henrik Quanz, Georg Albrecht, Christian Logemann, Derck Schlettwein, Peter R. Schreiner and Hermann A. Wegner
Angew. Chem. Int. Ed. 2019, 58, 4259–4263. DOI: 10.1002/anie.201814104
We present a new concept to control the conformations of molecules in the excited state through harvesting negative hyperconjugation. The strategy was realized with the 2,3,1,4‐benzodiazadiborinane scaffold, which was prepared by a new synthetic procedure. Photochemical studies identified dual light emission, which was assigned to well‐defined conformers. The emission at longer wavelength can be switched off by restricting the rotational degrees of freedom in the solid state as well as by controlling the energy levels of the excited states through adjusting the solvent polarity.
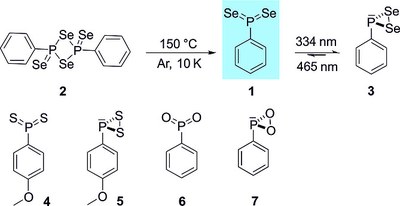
Preparation and Characterization of Phenyl Phosphine Diselenide – The Monomeric Form of Woollins' Reagent
Artur Mardyukov, Felix Keul and Peter R. Schreiner
Eur. J. Org. Chem. 2019, 46, 387-390. DOI: 10.1002/ejoc.201800639
We report the preparation, matrix‐isolation, and IR and UV/Vis spectroscopic characterization of phenyl phosphine diselenide, thus providing the first experimental evidence of the monomeric form of Woollins' reagent. Phenyl phosphine diselenide was prepared by thermal dissociation of Woollins' reagent and was identified by matching its spectroscopic data with density functional theory [B3LYP‐D3/6‐311++G(3df,3pd)] computations. The title compound proved to be highly photolabile and irradiation with light at λ = 334 nm results in the formation of hitherto unknown phenyldiselenyl phosphirane. Upon λ = 465 nm irradiation it rearranges back to phenyl phosphine diselenide.
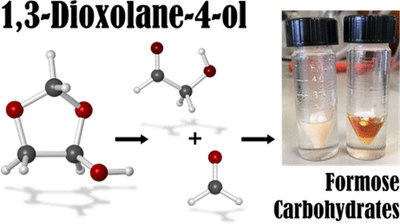
1,3-Dioxolane-4-ol Hemiacetal Stores Formaldehyde and Glycolaldhyde in the Gas Phase
André K. Eckhardt, Raffael C. Wende and Peter R. Schreiner
J. Am. Chem. Soc. 2018, 140,12333–12336. DOI: 10.1021/jacs.8b07480. Highlight: Cover picture of this issue.
We report the spontaneous gas-phase formation of 1,3-dioxolane-4-ol, a mixed hemiacetal resulting from the addition of glycolaldehyde to formaldehyde. It was spectroscopically characterized by matching matrix IR spectra with coupled cluster computations. The formation of the hemiacetal must be surface-catalyzed owing to the very high computed reaction barrier of 39.8 kcal mol–1. The reaction barrier is lowered by almost 20 kcal mol–1 when a single water molecule acts as a proton shuttle in a favorable six-membered transition state. We characterized the hemiacetal in solution via NMR spectroscopy and followed its decomposition into its constituents within a few hours; it also dissociates upon contact with water. Sugars form in the presence of Ca(OH)2, in line with formose-type reactivity. 1,3-Dioxolane-4-ol may be considered a storage form for formaldehyde and glycolaldehyde that is rather stable in the gas-phase.
Phenylsulfinyl Radical: Gas-Phase Generation, Photoisomerization and Oxidation
Jian Xu, Zhuang Wu, Huabin Wan, Guohai Deng, Bo Lu, André K. Eckhardt, Peter R. Schreiner, Tarek Trabelsi, Joseph S. Francisco and Xiaoqing Zeng
J. Am. Chem. Soc. 2018, 140, 9972–9978. DOI: 10.1021/jacs.8b05055
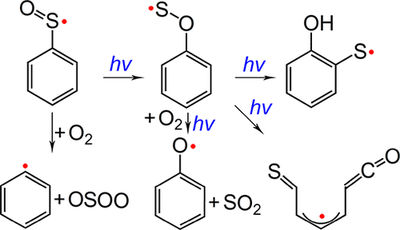
Arylsulfinyl radicals are key intermediates in sulfoxide chemistry. The parent molecule, phenylsulfinyl radical PhSO•, has been generated for the first time in the gas phase through high-vacuum flash pyrolysis of PhS(O)R (R = CF3 and Cl) at about 1000 K. Upon UV light irradiation (365 nm), PhSO• isomerizes to novel oxathiyl radical PhOS• in cryogenic matrices (2.8 K). Prolonged irradiation causes further isomerization of PhOS• to 2-hydroxyphenylthiyl radical, the formation of which has been also observed in the 193 nm laser photolysis of matrix-isolated 2-hydroxybenzenethiol. Concomitantly, ring-opening occurs during the UV photolysis of PhOS• and 2-hydroxybenzenethiol and forms an acyclic thioketoketene radical. Phenylsulfinyl radical reacts partially with molecular oxygen in the gas phase and yields phenyl radical Ph• and OSOO. Upon irradiation (365 nm), the isomeric oxathiyl radical also combines O2 with immediate dissociation to phenoxy radical PhO• and SO2. The identification of the intermediates with IR and UV–vis spectroscopy is supported by quantum chemical computations at the B3LYP/def2-TZVPP and UCCSD(T)/aug-cc-pV(D+d)Z levels of theory. The isomerization of PhSO• has been discussed based on the computed potential energy profile and the comparison with the intensively explored photochemistry of phenylperoxy radical PhOO•.
Making Glycine Methyl Ester Chiral
Dennis Gerbig, Sarina Desch and Peter R. Schreiner
Chem. Eur. J. 2018, 24, 11904-11907. DOI: 10.1002/chem.201802119
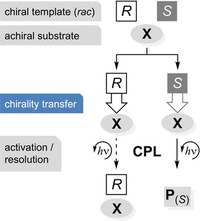
We demonstrate that the simple achiral amino acid glycine as its methyl ester inherits the chiral imprint of methyl lactate upon complexation, resulting in induced vibrational optical activity of the methylene C−H bonds. To mimic conditions of ice on comets that are considered long‐term reaction as well as storage entities for (organic) molecules, we employ the matrix isolation technique in conjunction with vibrational circular dichroism spectroscopy and DFT computations. The observed chirality transfer is likely a key element for the realization of concepts rationalizing chirogenesis, that is, the generation of a chiral imbalance.
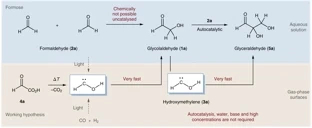
Gas-phase sugar formation using hydroxymethylene as the reactive formaldehyde isomer.
André K. Eckhardt, Michael M. Linden, Raffael C. Wende, Bastian Bernhardt and Peter R. Schreiner
Nature Chem. 2018, 10, 1141–1147. DOI: 10.1038/s41557-018-0128-2
Carbohydrates (CH2O)n are the formal adducts of carbon (atoms) to water with a repeating unit that structurally resembles H–C̈–OH (hydroxymethylene). Although hydroxymethylene has been suggested as a building block for sugar formation, it is a reactive species that had escaped detection until recently. Here we demonstrate that formaldehyde reacts with its isomer hydroxymethylene to give glycolaldehyde in a nearly barrierless reaction. This carbonyl–ene-type transformation operates in the absence of base and solvent at cryogenic temperatures similar to those found in extraterrestrial environments or interstellar clouds. Hydroxymethylene acts as a building block for an iterative sugar synthesis, as we demonstrate through the formation of the triose glyceraldehyde. The thermodynamically preferred ketose dihydroxyacetone does not form, and the formation of further branched sugars in the iterative synthesis presented here is unlikely. The results therefore provide a link between the well-known formose (Butlerow) reaction and sugar formation under non-aqueous conditions.
The Near-UV Absorber OSSO and its Isomers
Zhuang Wu, Huabin Wan, Jian Xu, Bo Lu, André K. Eckhardt, Peter R. Schreiner, Changjian Xie, Hua Guo and Xiaoqing Zeng
Chem. Commun. 2018, 54, 4517–4520. DOI: 10.1039/C8CC00999F
Highlight: Venusian near-UV absorber mystery solved in Chemistry World 2018, March 09, 2018.
Disulfur dioxide, OSSO, has been proposed as the enigmatic “near-UV absorber” in the yellowish atmosphere of Venus. However, the fundamentally important spectroscopic properties and photochemistry of OSSO are scarcely documented. By either condensing gaseous SO or 266 laser photolysis of an S2⋯O2 complex in Ar or N2 at 15 K, syn-OSSO, anti-OSSO, and cyclic OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O)S were identified by IR and UV/Vis spectroscopy for the first time. The observed absorptions (λmax) for OSSO at 517 and 390 nm coincide with the near-UV absorption (320–400 nm) found in the Venus clouds by photometric measurements with the Pioneer Venus orbiter. Subsequent UV light irradiation (365 nm) depletes syn-OSSO and anti-OSSO and yields a fourth isomer, syn-OSOS, with concomitant dissociation into SO2 and elemental sulfur.
O)S were identified by IR and UV/Vis spectroscopy for the first time. The observed absorptions (λmax) for OSSO at 517 and 390 nm coincide with the near-UV absorption (320–400 nm) found in the Venus clouds by photometric measurements with the Pioneer Venus orbiter. Subsequent UV light irradiation (365 nm) depletes syn-OSSO and anti-OSSO and yields a fourth isomer, syn-OSOS, with concomitant dissociation into SO2 and elemental sulfur.

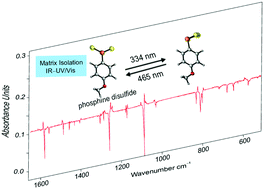
Unravelling Lawesson’s Reagent – The Structure of Monomeric (4-Methoxyphenyl)phosphine Disulfide
Artur Mardyukov, Dominik Niedek and Peter R. Schreiner
Chem. Commun. 2018, 54, 2715–2718. DOI: 10.1039/c8cc00034d
Highlights: a) Front cover of corresponding issue; b) “HotChem” article featured by the Royal Society of Chemistry.
We describe the isolation as well as IR and UV/Vis spectroscopic characterization of (4-methoxyphenyl)phosphine disulfide in argon matrices at 10 K. The title compound proved to be highly photolabile; irradiation with UV light (λ = 334 nm) led to rearrangement to the equally unreported 3-(4-methoxyphenyl)-1,2,3-dithiaphosphirane. Photoreversion can be achieved upon irradiation at λ = 465 nm.
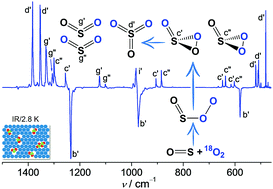
Capture of SO3 Isomers in the Oxidation of Sulfur Monoxide with Molecular Oxygen
Zhuang Wu, Bo Lu, Ruijuan Feng, Jian Xu, Yan Lu, Huabin Wan, André K. Eckhardt, Peter R. Schreiner, Changjian Xie, Hua Guo, and Xiaoqing Zeng
Chem. Commun. 2018, 54, 1690–1693. DOI: 10.1039/C7CC09389F
Highlight: Inside front cover.
When mixing SO with O2 in N2, Ne, or Ar, an end-on complex OS–OO forms in the gas phase and can subsequently be trapped at cryogenic temperatures (2.8–15.0 K). Upon infrared light irradiation, OS–OO converts to SO3 and SO2 + O with the concomitant formation of a rare 1,2,3-dioxathiirane 2-oxide, i.e., cyclic OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O)O. Unexpectedly, the ring-closure of 16OS–18O18O yields a ca. 2 : 1 mixture of cyclic 18OS(
O)O. Unexpectedly, the ring-closure of 16OS–18O18O yields a ca. 2 : 1 mixture of cyclic 18OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) 16O)18O and 16OS(
16O)18O and 16OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) 18O)18O. The characterization of OS–OO and OS(
18O)18O. The characterization of OS–OO and OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O)O with IR and UV/Vis spectroscopy is supported by high-level ab initio computations.
O)O with IR and UV/Vis spectroscopy is supported by high-level ab initio computations.
Atmospherically Relevant Radicals Derived from the Oxidation of Dimethyl Sulfide
Artur Mardyukov and Peter R. Schreiner
Acc. Chem. Res. 2018, 51, 475–483. DOI: 10.1021/acs.accounts.7b00536
The large number and amounts of volatile organosulfur compounds emitted to the atmosphere and the enormous variety of their reactions in various oxidation states make experimental measurements of even a small fraction of them a daunting task. Dimethyl sulfide (DMS) is a product of biological processes involving marine phytoplankton, and it is estimated to account for approximately 60% of the total natural sulfur gases released to the atmosphere. Ocean-emitted DMS has been suggested to play a role in atmospheric aerosol formation and thereby cloud formation. The reaction of ·OH with DMS is known to proceed by two independent channels: abstraction and addition. The oxidation of DMS is believed to be initiated by the reaction with  ·OH and NO3· radicals, which eventually leads to the formation of sulfuric acid (H2SO4) and methanesulfonic acid (CH3SO3H). The reaction of DMS with NO3· appears to proceed exclusively by hydrogen abstraction. The oxidation of DMS consists of a complex sequence of reactions. Depending on the time of the day or altitude, it may take a variety of pathways. In general, however, the oxidation proceeds via chains of radical reactions. Dimethyl sulfoxide (DMSO) has been reported to be a major product of the addition channel. Dimethyl sulfone (DMSO2), SO2, CH3SO3H, and methanesulfinic acid (CH3S(O)OH) have been observed as products of further oxidation of DMSO. Understanding the details of DMS oxidation requires in-depth knowledge of the elementary steps of this seemingly simple transformation, which in turn requires a combination of experimental and theoretical methods. The methylthiyl (CH3S·), methylsulfinyl (CH3SO·), methylsulfonyl (CH3SO2·), and methylsulfonyloxyl (CH3SO3·) radicals have been postulated as intermediates in the oxidation of DMS. Therefore, studying the chemistry of sulfur-containing free radicals in the laboratory also is the basis for understanding the mechanism of DMS oxidation in the atmosphere. The application of matrix-isolation techniques in combination with quantum-mechanical calculations on the generation and structural elucidation of CH3SOx (x = 0–3) radicals is reviewed in the present Account. Experimental matrix IR and UV/vis data for all known species of this substance class are summarized together with data obtained using other spectroscopic techniques, including time-resolved spectroscopy, electron paramagnetic resonance spectroscopy, and others. We also discuss the reactivity and experimental characterization of these species to illustrate their practical relevance and highlight spectroscopic techniques available for the elucidation of their geometric and electronic structures. The present Account summarizes recent results regarding the preparation, characterization, and reactivity of various radical species with the formula CH3SOx (x = 0–3).
·OH and NO3· radicals, which eventually leads to the formation of sulfuric acid (H2SO4) and methanesulfonic acid (CH3SO3H). The reaction of DMS with NO3· appears to proceed exclusively by hydrogen abstraction. The oxidation of DMS consists of a complex sequence of reactions. Depending on the time of the day or altitude, it may take a variety of pathways. In general, however, the oxidation proceeds via chains of radical reactions. Dimethyl sulfoxide (DMSO) has been reported to be a major product of the addition channel. Dimethyl sulfone (DMSO2), SO2, CH3SO3H, and methanesulfinic acid (CH3S(O)OH) have been observed as products of further oxidation of DMSO. Understanding the details of DMS oxidation requires in-depth knowledge of the elementary steps of this seemingly simple transformation, which in turn requires a combination of experimental and theoretical methods. The methylthiyl (CH3S·), methylsulfinyl (CH3SO·), methylsulfonyl (CH3SO2·), and methylsulfonyloxyl (CH3SO3·) radicals have been postulated as intermediates in the oxidation of DMS. Therefore, studying the chemistry of sulfur-containing free radicals in the laboratory also is the basis for understanding the mechanism of DMS oxidation in the atmosphere. The application of matrix-isolation techniques in combination with quantum-mechanical calculations on the generation and structural elucidation of CH3SOx (x = 0–3) radicals is reviewed in the present Account. Experimental matrix IR and UV/vis data for all known species of this substance class are summarized together with data obtained using other spectroscopic techniques, including time-resolved spectroscopy, electron paramagnetic resonance spectroscopy, and others. We also discuss the reactivity and experimental characterization of these species to illustrate their practical relevance and highlight spectroscopic techniques available for the elucidation of their geometric and electronic structures. The present Account summarizes recent results regarding the preparation, characterization, and reactivity of various radical species with the formula CH3SOx (x = 0–3).
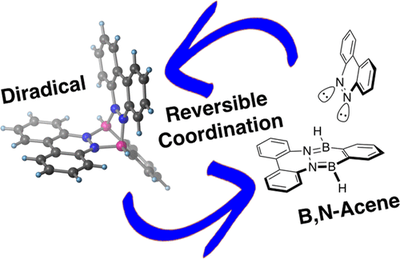
A Stable Organic Neutral Diboron Diradical via Reversible Coordination
Zhenpin Lu, Henrik Quanz, Olaf Burghaus, Jonas Hoffmann, Christian Logemann, Peter R. Schreiner, Sebastian Beeck and Hermann A. Wegner
J. Am. Chem. Soc. 2018, 139, 18488–18491. DOI: 10.1021/jacs.7b11823
We report the formation of a stable neutral diboron diradical simply by coordination of an aromatic dinitrogen compound to an ortho-phenyldiborane. This process is reversible upon addition of pyridine. The diradical species is stable above 200 °C. Computations are consistent with an open-shell triplet diradical with a very small open-shell singlet–triplet energy gap that is indicative of the electronic disjointness of the two radical sites. This opens a new way of generating stable radicals with fascinating electronic properties useful for a large variety of applications.
The Trifluoromethyl Sulfinyl and Oxythiyl Radicals
Zhuang Wu, Jian Xu, Guohai Deng, Yan Lu, Liubov Sokolenko, Tarek Trabelsi, Joseph S. Francisco, André Kristopher Eckhardt, Peter R. Schreiner and Xiaoqing Zeng
Chem. Eur. J. 2018, 24, 1505-1508. DOI: 10.1002/chem.201705142
Highlight: Cover feature DOI: 10.1002/chem.201705533

The trifluoromethyl sulfinyl radical and the trifluoromethyl oxathiyl radical, were generated in the gas phase and then trapped in solid noble gas matrices for characterization with IR and UV/Vis spectroscopy. Subsequent UV light irradiation resulted in an unprecedented isomerization from a sulfinyl radical to an oxathiyl radical by surmounting a formidable activation barrier, despite that such geometrical transformation has been computationally predicted for decades.
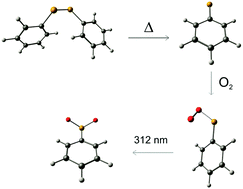
The Phenylselenyl Radical and Its Reaction with Molecular Oxygen
Artur Mardyukov,Yetsedaw Tsegaw, Wolfram Sander and Peter R. Schreiner
PCCP 2017, 19, 27384-27388. DOI: 10.1039/C7CP05546C
The current study focuses on the generation, identification, and characterization of the phenylselenyl radical using the matrix isolation technique in combination with density functional theory (B3LYP/cc-pVTZ) computations. The hitherto unknown phenylselenyl peroxy radical was synthesized by co-condensation of the phenylselenyl radical with molecular ground state triplet oxygen from the gas phase and subsequent trapping in argon matrices at 10 K. The experimental IR spectra including 18O isotopically labelled materials compare well with the data obtained from B3LYP/cc-pVTZ computations. Upon 312 nm irradiation, the phenylselenyl peroxy radical isomerizes to the thermodynamically more stable equally novel phenylselenoyl radical.
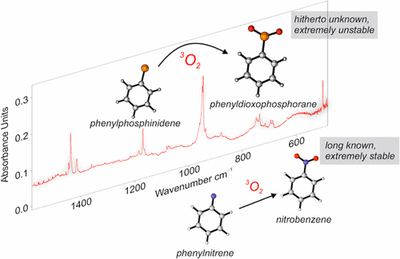
Preparation and Characterization of Parent Phenylphosphinidene and its Oxidation to Phenyldioxophosphorane, the Elusive Phosphorous Analogue of Nitrobenzene
Artur Mardyukov, Dominik Niedek and Peter R. Schreiner
J. Am. Chem. Soc. 2017, 139,5019–5022. DOI: 10.1021/jacs.7b01639
Triplet phenylphosphinidene was prepared by light-induced elimination of ethylene from the corresponding phenylphosphirane and was characterized by IR and UV/vis spectroscopy together with matching of its spectral data with density functional theory computations. The photolysis of phenylphosphirane in 3P-O2 doped matrices enabled the spectroscopic identification of a hitherto unknown phenyldioxophosphorane, the long elusive phosphorus analogue of nitrobenzene.
Towards the Pyrolytic Preparation of Carbonothioic O,O-Acid (Monothiocarbonic Acid)
Dominik Niedek, J. Philipp Wagner, and Peter R. Schreiner*
J. Anal. Appl. Pyrol. 2017, 124, 439–445. DOI: 10.1016/j.jaap.2017.02.024
Carbonothioic O,O-acid (monothiocarbonic acid), the sulfur analogue of carbonic acid, is thus far an experimentally unreported molecule of atmospheric and biochemical relevance. Computations at the CCSD(T)/cc-pVQZ//CCSD(T)/cc-pVTZ level of theory reveal that its carbonothioic O,S-acid tautomer is energetically favored over the carbonothioic O,O-acid tautomer and that the decomposition of the former to H2S and CO2 is prevented by a barrier in excess of 30 kcal mol⁻¹. Therefore, we attempted to prepare carbonothioic-O,O-acid under matrix isolation conditions via high vacuum flash pyrolysis (HVFP) of O,S-di-tert-butyl carbonothioate in analogy to the previously reported successful preparation of carbonic acid. Pyrolysis at 900 °C only produced isobutene, CO2, and tert-buthylthiol, while S-tert-butyl O-hydrogen carbonothioate is the most likely additional product identifiable at 690 °C pyrolysis temperature. Density functional theory computations at the M06-2X/cc-pVTZ level were used to understand the pyrolysis pathways and help determine the leaving group ability in the ester pyrolysis step, and our inability to observe the carbonothioic acids via our chosen pyrolysis path.
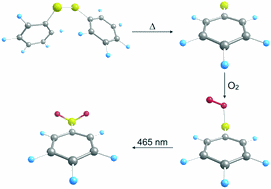
Generation and Characterization of the Phenylthiyl Radical and its Oxidation to the Phenylthiylperoxy and Sulfonylbenzenyl Radical
Artur Mardyukov and Peter R. Schreiner*
PCCP 2016, 18, 26161–26165. DOI: 10.1039/C6CP04278C
The phenylthiyl radical (1) was prepared in the gas phase by vacuum flash pyrolysis of allylphenyl sulfide or diphenyl sulfide and isolated in an argon matrix. The hitherto unknown phenylthiyl peroxy radical was synthesized by co-condensation of 1 with molecular oxygen. Irradiation with light of λ = 465 nm led to a rearrangement to the novel phenylsulfonyl radical.
Forming Stereogenic Centers in Acyclic Systems from Alkynes
Roxane Vabre, Biana Island, Claudia Diehl, Peter R. Schreiner, and Ilan Marek*
Angew. Chem. Int. Ed. 2015, 54, 9996–9999. DOI: 10.1002/anie.201504756
Noted as very important paper (top 5% of all Angewandte publications)
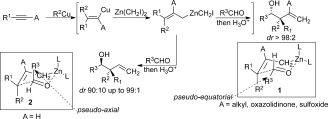
The combined carbometalation/zinc homologation followed by reactions with α-heterosubstituted aldehydes and imines proceed through a chair-like transition structure with the substituent of the incoming aldehyde residue preferentially occupying a pseudo-axial position to avoid the two gauche interactions. The heteroatom in the axial position produces a chelated intermediate (and not a Cornforth–Evans transition structure for α-chloro aldehydes and imines) leading to a face differentiation in the allylation reaction. This method provides access to functionalized products in which three new carbon–carbon bonds and two to three stereogenic centers, including a quaternary one, were created in acyclic systems in a single-pot operation from simple alkynes.