Capture of SO3 Isomers in the Oxidation of Sulfur Monoxide with Molecular Oxygen
Zhuang Wu, Bo Lu, Ruijuan Feng, Jian Xu, Yan Lu, Huabin Wan, André K. Eckhardt, Peter R. Schreiner, Changjian Xie, Hua Guo, and Xiaoqing Zeng
Chem. Commun. 2018, 54, 1690–1693. DOI: 10.1039/C7CC09389F
Highlight: Inside front cover.
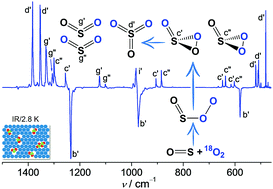
When mixing SO with O2 in N2, Ne, or Ar, an end-on complex OS–OO forms in the gas phase and can subsequently be trapped at cryogenic temperatures (2.8–15.0 K). Upon infrared light irradiation, OS–OO converts to SO3 and SO2 + O with the concomitant formation of a rare 1,2,3-dioxathiirane 2-oxide, i.e., cyclic OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O)O. Unexpectedly, the ring-closure of 16OS–18O18O yields a ca. 2 : 1 mixture of cyclic 18OS(
O)O. Unexpectedly, the ring-closure of 16OS–18O18O yields a ca. 2 : 1 mixture of cyclic 18OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) 16O)18O and 16OS(
16O)18O and 16OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) 18O)18O. The characterization of OS–OO and OS(
18O)18O. The characterization of OS–OO and OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O)O with IR and UV/Vis spectroscopy is supported by high-level ab initio computations.
O)O with IR and UV/Vis spectroscopy is supported by high-level ab initio computations.