Dispersion
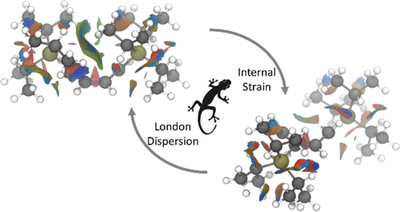
A Gecko can walk up a smooth glass window because of the adhesion in the millions of hydrophobic setae on its toes that convey van der Waals (vdW) interactions with the surface. The attractive part of such vdW-interactions is an electron correlation (quantum mechanical many-body) effect normally referred to as London dispersion. Its overwhelming role in the formation of condensed matter has been known since the pioneering contributions of J. D. van der Waals and F. London who related dispersion to spectroscopic properties, particularly the polarizability of the interacting partners. In chemistry, dispersion helps rationalize the varying boiling points of alkanes, the greater stability of branched over linear alkanes, the π-π attractive structures of graphite and graphenes, and the mutual attraction of s-π systems. Strong covalent bonding as well as non-covalent interactions such as hydrogen- or halogen-bonding, Pauli repulsion (“steric effects”), and electrostatic multipole interactions are mainly used as “design elements” to affect chemical structures and reactivity. Extending this incomplete view is one of the key goals of the Schreiner Group, with a focus on dispersion interactions as important control elements for structure and reactivity of organic molecules. One of the major objectives is the qualitative determination and quantification of dispersion interactions utilizing a selection of particularly suitable molecular systems. A selection of our work in this arena can be found below.
- Director: Univ.-Prof. Peter R. Schreiner
-
Co-workers:
- Dispersion
-
Current work:
DFG priority program "SPP 1807 - Control of London dispersion interactions in molecular chemistry"
Exploring the Limits of Intramolecular London Dispersion Stabilization with Bulky Dispersion Energy Donors in Solution. Functionalized Nanodiamonds, part 94. Jan M. Schümann, Lukas Ochmann, Jonathan Becker, Ahmet Altun, Ingolf Harden, Giovanni Bistoni and Peter R. Schreiner
Am. Chem. Soc. 2023, 145, 2093–2097. DOI: 10.1021/jacs.2c13301.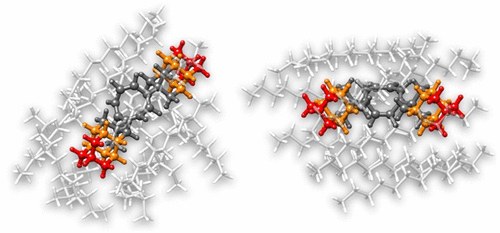
We present an experimental study of a cyclooctatetraene-based molecular balance disubstituted with increasingly bulky tert-butyl (tBu), adamantyl (Ad), and diamantyl (Dia) substituents in the 1,4-/1,6-positions for which we determined the valence-bond shift equilibrium in n-hexane (hex), n-octane (oct), and n-dodecane (dod). Computations including implicit and explicit solvation support our temperature-dependent NMR equilibrium measurements indicating that the more sterically crowded 1,6-isomer is always favored, irrespective of solvent, and that the free energy is quite insensitive to substituent size.
Probing the Size-Limit of Dispersion Energy Donors with a Bifluorenylidene Balance: Magic Cyclohexyl. Finn M. Wilming, Benito Marazzi, Paul P. Debes, Jonathan Becker, and Peter R. Schreiner
Org. Chem. 2023, 88, 1024–1035. DOI: 10.1021/acs.joc.2c02444.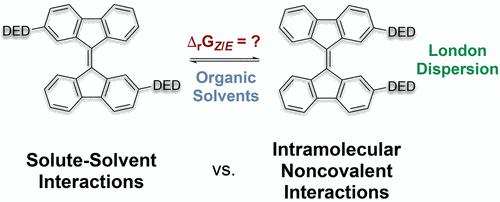
We report the synthesis of 14 2,2′-disubstituted 9,9′-bifluorenylidenes as molecular balances for the quantification of London dispersion interactions between various dispersion energy donors. For all balances, we measured ΔGZ/E at 333 K using 1H NMR in seven organic solvents. For various alkyl and aryl substituents, we generally observe a preference for the “folded” Z-isomer due to attractive London dispersion interactions. The cyclohexyl-substituted system shows the largest Z-preference in this study with ΔGZ/E = −0.6 ± 0.05 kcal mol–1 in all solvents, owing to the rotational freedom of cyclohexyl groups paired with their large polarizability that maximizes London dispersion interactions. On the other hand, rigid and sterically more demanding substituents like tert-butyl unexpectedly favor the unfolded E-isomer. This is a result of the close relative position in which the functional groups are positioned in this molecular balance. This close proximity is the reason for the increase of Pauli repulsion in the Z-isomers with large rigid substituents (tert-butyl, adamantyl, and diamantyl) which leads to an equilibrium shift toward the unfolded E-form. While we were able to reproduce most of our experimental trends qualitatively using contemporary computational chemistry methods, quantitative accuracy of the employed methods still needs further improvement.
All that metas – Synthesis of Dispersion Energy Donor-Substituted Benzenes. Lukas Ochmann, Michael Fuhrmann, Felix J. Gössl, Alexander Makaveev, and Peter R. Schreiner
Lett. 2022, 24, 6968–6972. DOI: 10.1021/acs.orglett.2c02780.
Highlight: Org. Process Res. Dev. 2022, 26, 2999.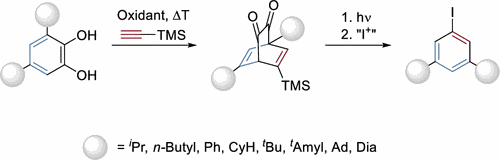
We report a general and scalable method for the synthesis of all-meta-trisubstituted benzenes from readily available 3,5-disubstituted catechols. Oxidation and [4 + 2] cycloaddition with acetylene dienophiles generate a bicyclo[2.2.2]octane structure that is doubly decarbonylated initiated by blue-light irradiation, leading to a meta,meta-disubstitution pattern on the re-aromatized system. This enables this substitution pattern even with very bulky alkyl groups (deemed excellent dispersion energy donors) to be incorporated into, for example, chiral phosphoric acid catalysts.
Silyl Groups are Strong Dispersion Energy Donors. Lars Rummel, Henrik F. König, Heike Hausmann, and Peter R. Schreiner
Org. Chem. 2022, 87, 13168–13177. DOI: 10.1021/acs.joc.2c01633.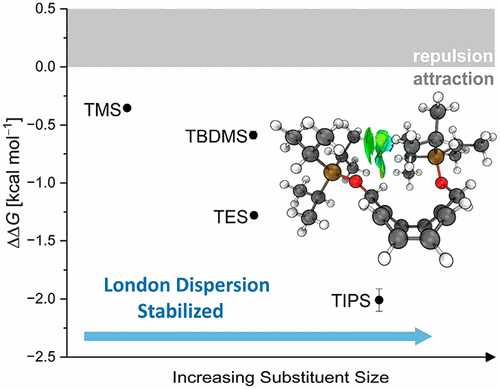
We present an experimental and computational study to investigate noncovalent interactions between silyl groups that are often employed as “innocent” protecting groups. We chose an extended cyclooctatetraene (COT)-based molecular balance comprising unfolded (1,4-disubstituted) and folded (1,6-disubstituted) valance bond isomers that typically display remote and close silyl group contacts, respectively. The thermodynamic equilibria were determined using nuclear magnetic resonance measurements. Additionally, we utilized Boltzmann weighted symmetry-adapted perturbation theory (SAPT) at the sSAPT0/aug-cc-pVDZ level of theory to dissect and quantify noncovalent interactions. Apart from the extremely bulky tris(trimethylsilyl)silyl “supersilyl” group, there is a preference for the folded 1,6-COT valence isomer, with London dispersion interactions being the main stabilizing factor. This makes silyl groups excellent dispersion energy donors, a finding that needs to be taken into account in synthesis planning.
London Dispersion Stabilizes Chloro-Substituted cis-Double Bonds. Lars Rummel, Kai Hanke, Jonathan Becker, and Peter R. Schreiner
Synlett 2022, 34, 1129–1134. DOI: 10.1055/a-1928-2473.We present a combined experimental and computational study on the thermodynamic stability of cis- and trans-alkenes substituted with dispersion energy donor (DED) groups. To investigate the role of noncovalent interactions on equilibrium of cis- and trans-alkenes we utilized hydrochlorination reactions. While the general assumption is that increasing steric bulk favors the trans-alkene, we observe an equilibrium shift towards the more crowded cis-alkene with increasing substituent size. With the aim to quantify noncovalent interactions, we performed a double mutant cycle to experimentally gauge the attractive potential of bulky substituents. Additionally, we utilized local energy decomposition analysis at the DLPNO-CCSD(T)/def2-TZVP level of theory. We found LD interactions and Pauli exchange repulsion to be the most dominant components to influence cis- and trans-alkene equilibria.
Assessing the Experimental Hydrogen Bonding Energy of the Cyclic Water Dimer Transition State with a Cyclooctatetraene-Based Molecular Balance. Henrik F. König, Heike Hausmann, and Peter R. Schreiner
Am. Chem. Soc. 2022, 144, 16965–16973. DOI: 10.1021/jacs.2c06141.
Highlight: Selected as front cover picture of this issue (37).
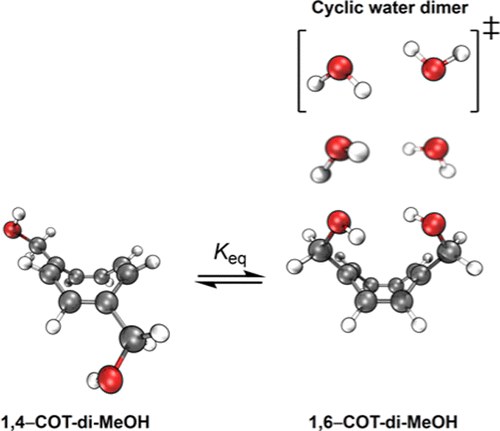
We have conducted an experimental and computational study of cyclooctatetraene-1,4/1,6-dimethanol (1,4 and 1,6) as a molecular balance with the goal in mind to determine the otherwise inaccessible hydrogen bonding energy (HBE) of the cyclic water dimer, which constitutes a transition state. The 1,4/1,6 folding equilibrium is governed by an intramolecular hydrogen bond in the folded 1,6-isomer, in which the OH groups adopt a cyclic planar geometry, akin to the structure of the cyclic water dimer transition state. We characterized hydrogen bonding in 1,6 and reference complexes utilizing SAPT2 + (3)δMP2/aug-cc-pVTZ and selected quantum theory of atoms in molecule descriptors at M06-2XD3(0)/ma-def2-TZVPP. Additionally, we computed HBEs at the DLPNO-CCSD(T)/aug-cc-pVQZ level of theory. We find that hydrogen bonding in 1,6 is very similar to the interaction in the Ci symmetric cyclic water dimer TS, both in magnitude and character. We experimentally determined the Gibbs free energy of the folding process (ΔGeq) in a variety of organic solvents via nuclear magnetic resonance spectroscopy measurements at room temperature. By combining experimentally obtained ΔGeq values with corrections derived from accurate computational methods, we provide estimates for the HBE of cyclic water dimers and the cyclic water dimer TS, as the most stable cyclic water dimer.
London Dispersion Favors Sterically Hindered Diarylthiourea Conformers in Solution. Lars Rummel, Marvin H. J. Domanski, Heike Hausmann, Jonathan Becker, and Peter R. Schreiner
Chem. Int. Ed. 2022, 61, e202204393. DOI: 10.1002/anie.202204393.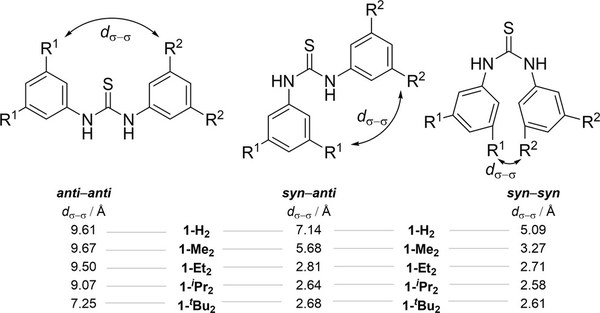
We present an experimental and computational study on the conformers of N,N′-diphenylthiourea substituted with different dispersion energy donor (DED) groups. While the unfolded anti–anti conformer is the most relevant for thiourea catalysis, intramolecular noncovalent interactions counterintuitively favor the folded syn–syn conformer, as evident from a combination of low-temperature nuclear magnetic resonance measurements and computations. In order to quantify the noncovalent interactions, we utilized local energy decomposition analysis and symmetry-adapted perturbation theory at the DLPNO-CCSD(T)/def2-TZVPP and sSAPT0/6-311G(d,p) levels of theory. Additionally, we applied a double-mutant cycle to experimentally study the effects of bulky substituents on the equilibria. We determined London dispersion as the key interaction that shifts the equilibria towards the syn–syn conformers. This preference is likely a factor why such thiourea derivatives can be poor catalysts.
Computational Chemistry as a Conceptual Game Changer: Understanding the Role of London Dispersion in Hexaphenylethane Derivatives (Gomberg Systems). Sören Rösel and Peter R. Schreiner
Isr. J. Chem. 2022, 62, e202200002. DOI: 10.1002/ijch.202200002.The present personal perspective sheds light on the checkered history of hexaphenylethane (HPE) and some of its key derivatives, including successes and failures in interpreting experimental as well as computational data. HPE has become a testing ground for chemical theory since the groundbreaking work of Gomberg in 1900. It sparked the growth of theoretical carbon chemistry beyond tetravalency, forced chemists to improve theory of color and chemical bonding by mesomeric resonance, and challenged the purely repulsive view on substituent effects. Understanding the origin of the instability of HPE on the one hand and the stability of the sterically much more crowded all-meta tBu-substituted HPE on the other hand may well be considered an important turning point in the appreciation of London dispersion (LD) and the analytical power of computational chemistry.
Gauging the Steric Effects of Silyl Groups with a Molecular Balance. Henrik F. König, Lars Rummel, Heike Hausmann, Jonathan Becker, Jan M. Schümann and Peter R. Schreiner
J. Org. Chem. 2022, 87, 4670–4679. DOI: 10.1021/acs.joc.1c03103.We present an experimental and computational study of a cyclooctatetraene (COT)-based molecular balance disubstituted with commonly used silyl groups. Such groups often serve as protecting groups and are typically considered innocent bystanders. Our motivation here is to determine the actual steric effects of such groups by employing a molecular balance. While in the unfolded 1,4-valence isomer the silyl groups are far apart (dσ–σ ≥ 5.15 Å), the folded 1,6-isomer is affected greatly by noncovalent interactions due to close σ–σ contacts (dσ–σ ≤ 2.58 Å). In order to investigate the thermodynamic equilibrium between the 1,6- and 1,4-valence isomers, we employed temperature-dependent nuclear magnetic resonance measurements. Additionally, we assessed the nature of attractive and repulsive interactions in 1,6-disilyl-COT derivatives via a combination of local energy decomposition analysis (LED) and symmetry-adapted perturbation theory (SAPT) at the DLPNO-CCSD(T)/def2-TZVP and sSAPT0/aug-cc-pVDZ levels of theory. We identified London dispersion interactions as the main contributor to the molecular stability of the folded states, whereas Pauli exchange repulsion and a resulting internal strain favor the unfolded diastereomer.
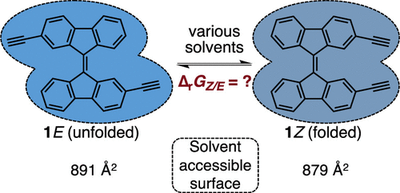
Quantifying Solvophobic Effects in Organic Solvents using a Hydrocarbon Molecular Balance. Finn M. Wilming, Jonathan Becker and Peter R. Schreiner
J. Org. Chem. 2022, 87, 1874–1878. DOI: 10.1021/acs.joc.1c01813.We evaluate the use of the cohesive energy density (ced) as a quantitative descriptor for solvophobic effects in organic solvents by measuring ΔGZ/E of the rigid Z- and E-2,2′-diethynyl-9,9′-bifluorenylidene. In line with previously employed balances, solvent-dependent changes in ΔGZ/E are predominantly induced by solvophobic effects, leading to a strong correlation with the solvent’s ced. We re-emphasize the role of ceds as quantitative descriptors of solvophobic effects of organic solvents. Our experimental findings are well supported by B3LYP-D3/def2TZVP computations.
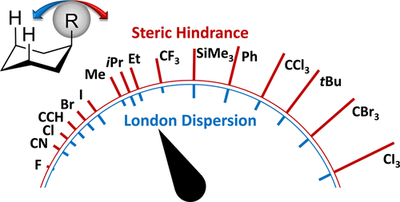
London Dispersion Helps Refine Steric A-Values: Dispersion Energy Donor Scales. Ephrath Solel, Marcel Ruth and Peter R. Schreiner
J. Am. Chem. Soc. 2021, 143, 20837–28848. DOI: 10.1021/jacs.1c09222.We suggest a scale of dispersion energy donors (DEDs) that allows for direct comparisons with steric effects. This scale is based on the classic A-values and allows groups to reorient to minimize strain, thereby providing an advantage over raw group polarizabilities. The A-value can no longer be considered purely a steric factor. Even for groups that do not participate in charge transfer or electrostatic interactions, the A-value includes Pauli repulsion (steric hindrance) and attractive London dispersion (LD) interactions. Although the common assumption is that, at the distances found in monosubstituted cyclohexanes, steric demands are the key factors influencing conformer preferences, we show in this computational study that there is a non-negligible LD part. We use this system to build a DED scale and a complementary steric scale. These scales are quantitatively comparable, as they are based on the same system, and allow for comparison of the two competing interactions in experimentally relevant settings. In addition, we show that LD interactions can be used to explain puzzling data regarding relative group sizes.
Hexaphenylditetrels – When Longer Bonds Provide Higher Stability. Lars Rummel, Jan M. Schümann and Peter R. Schreiner
Chem. Eur. J. 2021, 27, 13699–13702. DOI: 10.1002/chem.202102271. Highlight: Featured in Chemviews September 21, 2021.Carbon-Carbon bonds are exceptional as demonstrated for the hexaphenylditetrels where attractive London dispersion interactions are the decisive factor for the thermodynamic stabilities of tetrels other than carbon. Structural and energetic comparisons show that even though hexaphenylethane displays the largest dispersion energy between the two molecular halves, it has remained elusive because of even larger Pauli repulsion of the phenyl moieties that are forced in close contact due to the C−C bond that is the shortest in this series.
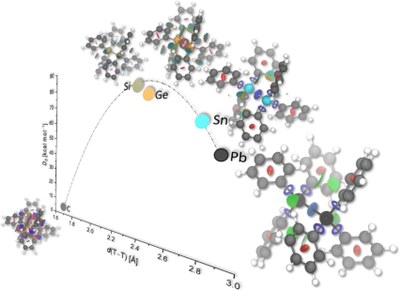
We present a computational analysis of hexaphenylethane derivatives with heavier tetrels comprising the central bond. In stark contrast to parent hexaphenylethane, the heavier tetrel derivatives can readily be prepared. In order to determine the origin of their apparent thermodynamic stability against dissociation as compared to the carbon case, we employed local energy decomposition analysis (LED) and symmetry-adapted perturbation theory (SAPT) at the DLPNO-CCSD(T)/def2-TZVP and sSAPT0/def2-TZVP levels of theory. We identified London dispersion (LD) interactions as the decisive factor for the molecular stability of heavier tetrel derivatives. This stability is made possible owing to the longer (than C−C) central bonds that move the phenyl groups out of the heavily repulsive regime so they can optimally benefit from LD interactions.
London Dispersion Helps Refine Steric A-Values: The Halogens. Ephrath Solel, Marcel Ruth, and Peter R. Schreiner
J. Org. Chem. 2021, 86, 7701–7713. DOI: 10.1021/acs.joc.1c00767Halogens are rarely considered as dispersion energy donors for organic reaction design. Here, we re-examine one of the textbook examples for assessing steric hindrance, the A-value, and demonstrate that even in this system, halogens cannot be treated solely as classic repulsive hard spheres. A significant part of the steric demand of the halogens is compensated by attractive London dispersion (LD) interactions, explaining the experimental lack of a clear trend when going down the halogens’ row. Beyond monohalogenated cyclohexanes, dihalo- and perhalocyclohexanes also show significant LD interactions. We also explored several other small organic systems containing halogens. Our findings show that organic chemists should treat halogens as possible sources of LD interactions in reaction design, as these atoms can change the landscape of the potential energy surface and reverse trends of conformer stabilities and reaction selectivities.
Direct Observation of the Shortest Intermolecular C–H…H–C Distance in a Dispersion-Bound Dimer in the Gas Phase via Stimulated Raman Spectroscopy. Dominique Maué, Patrick H. Strebert, Dominic Bernhard, Sören Rösel, Peter R. Schreiner and Markus Gerhards†
Angew. Chem. Int. Ed. 2021, 60, 11305–11309. DOI: 10.1002/anie.202016020. (†deceased 28.12.2020). Highlights: a) Noted as very important paper (top 5% of all Angewandte publications).Head-on Collision: Isolated gas phase triphenylmethane based dimers containing dispersion energy donors form a head-to-head structure with an extremely short C−H⋅⋅⋅H−C distance with a collinear arrangement of atoms. This surprising result from molecular beam experiments indicates that it is London dispersion but not crystal packing, responsible for this peculiar structural arrangement.
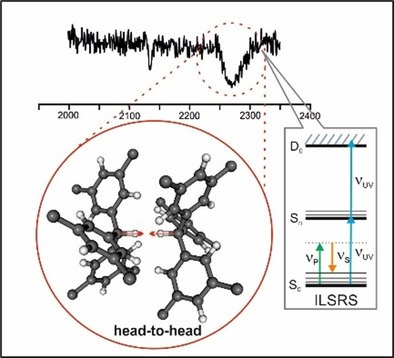
The triphenylmethane and all-meta tert-butyl triphenylmethane dimers, (TPM)2 and (T tBuPM)2, respectively, were studied with ionization loss stimulated Raman spectroscopy in molecular beam experiments to resolve structure sensitive vibrations. This answers the question whether the recently reported linear head-to-head arrangement in (T tBuPM)2 results from crystal packing or prevails also in the gas phase, and therefore must result from extraordinarily strong London dispersion (LD) interactions. Our study clearly demonstrates that the head-to-head arrangement is maintained even under isolated molecular beam conditions in the absence of crystal packing effects. The central Raman-active aliphatic C−D vibration of appropriately deuterated (T tBuPM)2 associated with an unusually short C−D⋅⋅⋅D−C distance exhibits a strong blue-shift compared to the undisturbed case. As the LD stabilizing tert-butyl groups are absent in (TPM)2, it displays an approximately S6-symmetric tail-to-tail arrangement.
Do docking sites persist upon fluorination? The diadamantyl ether-aromatics challenge for rotational spectroscopy and theory. María Mar Quesada Moreno, Pablo Pinacho, Cristóbal Pérez, Marina Šekutor, Peter R. Schreiner and Melanie Schnell
Chem. Eur. J. 2021, 28, 6198–6203. DOI: 10.1002/chem.202100078The effect of H→F substitution on the interactions and structure of complexes between diadamantyl ether and benzene and a series of fluorinated benzenes have been studied using rotational spectroscopy. London dispersion is the governing factor in directing spatial arrangement. The preferred binding position changes upon partial or full substitution, whereas the overall contributions of the intermolecular interactions involved are similar.
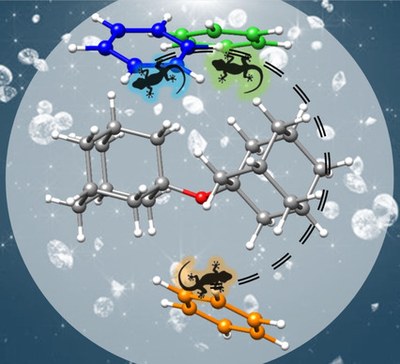
Fluorinated derivatives of biological molecules have proven to be highly efficient at modifying the biological activity of a given protein through changes in the stability and the kind of docking interactions. These interactions can be hindered or facilitated based on the hydrophilic/hydrophobic character of a particular protein region. Diadamantyl ether (C20H30O) possesses both kinds of docking sites, serving as a good template to model these important contacts with aromatic fluorinated counterparts. In this work, an experimental study on the structures of several complexes between diadamantyl ether and benzene as well as a series of fluorinated benzenes is reported to analyze the effect of H→F substitution on the interaction and structure of the resulting molecular clusters using rotational spectroscopy. All experimentally observed complexes are largely dominated by London dispersion interactions with the hydrogen-terminated surface areas of diadamantyl ether. Already single substitution of one hydrogen atom with fluorine changes the preferred docking site of the complexes. However, the overall contributions of the different intermolecular interactions are similar for the different complexes, contrary to previous studies focusing on the difference in interactions using fluorinated and non-fluorinated molecules.
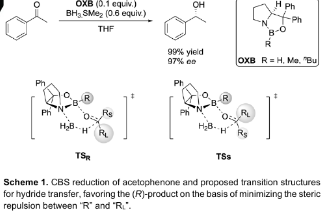
London Dispersion Rather than Steric Hindrance Determines the Enantioselectivity of the Corey-Bakshi-Shibata Reduction. Christian Eschmann, Lijuan Song, and Peter R. Schreiner
Angew. Chem. Int. Ed. 2021, 60, 4823-4832. DOI: 10.1002/anie.202012760The well‐known Corey‐Bakshi‐Shibata (CBS) reduction is a powerful method for the asymmetric synthesis of alcohols from prochiral ketones, often featuring high yields and excellent selectivities. While steric repulsion has been regarded as the key director of the observed high enantioselectivity for many years, here we show that London dispersion (LD) interactions are at least as important for enantiodiscrimination. We exemplify this through a combination of detailed computational and experimental studies for a series of modified CBS catalysts equipped with dispersion energy donors (DEDs) in the catalysts and the substrates. Our results demonstrate that attractive LD interactions between the catalyst and the substrate rather than steric repulsion determine the selectivity. As a key outcome of our study, we were able to improve the catalyst design for some challenging CBS reductions.
London Dispersion and Hydrogen‐Bonding Interactions in Bulky Molecules: The Case of Diadamantyl Ether Complexes. María Mar Quesada Moreno, Pablo Pinacho, Cristóbal Pérez, Marina Šekutor, Peter R. Schreiner and Melanie Schnell
Chem. Eur. J. 2020, 26, 10817–10825. DOI: 10.1002/chem.202001444Diadamantyl ether (DAE, C20H30O) represents a good model to study the interplay between London dispersion and hydrogen‐bond interactions. By using broadband rotational spectroscopy, an accurate experimental structure of the diadamantyl ether monomer is obtained and its aggregates with water and a variety of aliphatic alcohols of increasing size are analyzed. In the monomer, C−H⋅⋅⋅H−C London dispersion attractions between the two adamantyl subunits further stabilize its structure. Water and the alcohol partners bind to diadamantyl ether through hydrogen bonding and non‐covalent Owater/alcohol⋅⋅⋅H−CDAE and C−Halcohol⋅⋅⋅H−CDAE interactions. Electrostatic contributions drive the stabilization of all the complexes, whereas London dispersion interactions become more pronounced with increasing size of the alcohol. Complexes with dominant dispersion contributions are significantly higher in energy and were not observed in the experiment. The results presented herein shed light on the first steps of microsolvation and aggregation of molecular complexes with London dispersion energy donor (DED) groups and the kind of interactions that control them.
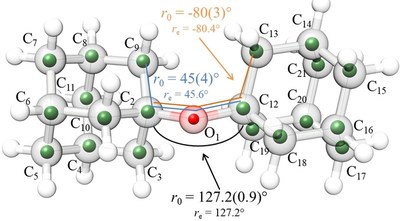
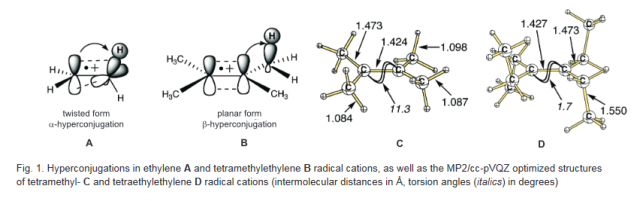
Noncovalent interactions in crowded olefinic radical cations. Andrey A. Fokin, Vladyslav Bakhonskyi, Tetyana V. Koso, Ngo Trung Hoc, Michael Serafin, Tatiana S. Zhuk, Vladimir N. Rodionov, Peter R. Schreiner
J. Org. Pharm. Chem. 2020, 18, 5–13. DOI: 10.24959/ophcj.20.189679Aim. To study the effect of electronic (α- and β-hyperconjugations) and steric (noncovalent interactions) factors on the structures of olefinic radical cations.
Results and discussion. The effect of intramolecular dispersion interactions on the structures of crowded alkenes in the neutral and ionized forms has been studied at the density functional theory (DFT) level with and without dispersion corrections included, as well as at the MP2 theory level with medium size basis sets. The results obtained are compared to the available experimental data. An excellent agreement has been found between the experimental and MP2/DFT-computed geometries of sesquihomoadamantene, adamantylidene adamantane, bis-2,2,5,5-tetramethylcyclopentylidene, bis-D3-homocub-4-ylidene, and bis-CS-homocub-8-ylidene in the neutral and ionized forms. The experimental ionization potentials are better reproduced with the DFT-methods.
Experimental part. The structure and composition of compounds were proved by the methods of 1H and 13C NMR-spectroscopy, and GC-MS-analysis. Elemental analysis was performed for the compounds obtained.
Conclusions. The twisting of the olefinic moieties in the sesquihomoadamantene and adamantylidene adamantane radical cations is determined by the balance between the σ-π-hyperconjugation and residual one-electron π-bonding and is close to that of the prototypical ethylene radical cation (29°). The twisting reaches 55° for the bis-2,2,5,5-tetramethylcyclopentylidene radical cation due to substantial steric repulsions between methyl groups. At the same time, the ionized states of bis-D3-homocub-4-ylidene and bis-CS-homocub-8-ylidene retain their planarity due to β-CC-hyperconjugation and intramolecular dispersion attractions.
The Role of London Dispersion Interactions in Ga‐Substituted Dipnictenes. Lijuan Song, Juliane Schoening, Christoph Wölper, Stephan Schulz and Peter R. Schreiner
Organometallics 2019, 38, 1640-1647. DOI: 10.1021/acs.organomet.9b00072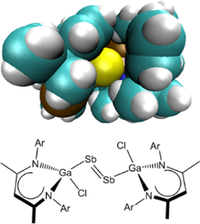
We report the synthesis and structural characterization of Ga-substituted diarsene [L(EtO)GaAs]2, which is an exemplary case of a Ga-substituted dipnictene of the general type [L(X)Ga]2E2 (L = C[C(Me)N(2,6-i-Pr2-C6H3)]2, X = F, Cl, Br, I, NMe2, OEt; E = As, Sb, Bi). We examined this extended series of compounds computationally and found that attractive London dispersion interactions between the substituents on the N,N′-chelating β-diketiminate ligands as well as pnictogen−π interactions between the group 15 metal center and the ligand are key factors for the stability and the electronic structures of such types of complexes.
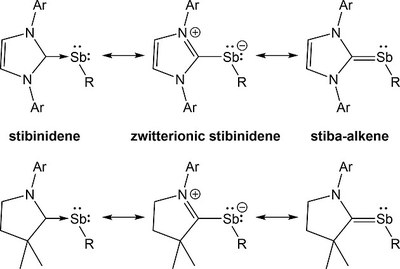
Syntheses, Structures and Bonding Analyses of Carbene-stabilized Stibinidenes. Julia Krüger, Christoph Wölper, Lukas John, Lijuan Song, Peter R. Schreiner, Stephan Schulz
Eur. J. Inorg. Chem. 2019, 1669–1678. DOI: 10.1002/ejic.201900167Reactions of two equivalents of LGa {L = HC[C(Me)NAr]2; Ar = 2,6‐iPr2‐C6H3} with SbX3 (X = Cl, Br) yield double‐inserted products (LGaX)2SbX (X = Cl 1, Br 2), which were isolated at –40 °C. Warming solutions of 1 and 2 to ambient temperature results in intramolecular elimination of LGaX2 and formation of Ga‐substituted stibinidenes L(X)GaSb as reaction intermediates, which were stabilized by coordination of strong σ‐donating carbenes. Four carbene‐stabilized stibinidenes MeCAAC‐SbGa(X)L {MeCAAC = [H2C(CMe2)2NAr]C, Ar = 2,6‐iPr2‐C6H3, X = Cl 3, Br 4} and IDipp‐SbGa(X)L {IDipp = 1,3‐bis(2,6‐diisopropylphenyl)‐imidazol‐2‐ylidene, X = Cl 5, Br 6} were characterized by single‐crystal X‐ray diffraction and the nature of the bonding in 3–6 was further investigated by quantum chemical methods. The studies reveal substantial Sb–Ccarbene π‐backbonding contributions in 3 and 4, whereas the IDipp‐stabilized derivatives 5 and 6 exhibit Sb–Ccarbene single bonds. DFT computations with and without dispersion corrections reveal that dispersion interactions mainly from the bulky aryl groups decisively contribute to the stability of 3–6.
Probing the Delicate Balance between Pauli Repulsion and London Dispersion with Triphenylmethyl Derivatives. Sören Rösel, Jonathan Becker, Wesley D. Allen and Peter R. Schreiner
J. Am. Chem. Soc. 2018, 140 (43), pp 14421–14432. DOI: 10.1021/jacs.8b09145. Highlight: JACS Spotlight .
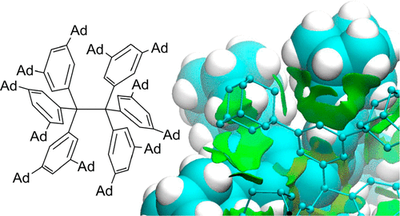
The long-known, ubiquitously present, and always attractive London dispersion (LD) interaction was probed with hexaphenylethane (HPE) derivatives. A series of all-meta hydrocarbyl [Me, iPr, tBu, Cy, Ph, 1-adamantyl (Ad)]-substituted triphenylmethyl (TPM) derivatives [TPM–H, TPM–OH, (TPM–O)2, TPM•] was synthesized en route, and several derivatives were characterized by single-crystal X-ray diffraction (SC-XRD). Multiple dimeric head-to-head SC-XRD structures feature an excellent geometric fit between the meta-substituents; this is particularly true for the sterically most demanding tBu and Ad substituents. NMR spectra of the iPr-, tBu-, and Cy-derived trityl radicals were obtained and reveal, together with EPR and UV–Vis spectroscopic data, that the effects of all-meta alkyl substitution on the electronic properties of the trityl scaffold are marginal. Therefore, we concluded that the most important factor for HPE stability arises from LD interactions. Beyond all-meta tBu-HPE we also identified the hitherto unreported all-meta Ad-HPE. An intricate mathematical analysis of the temperature-dependent dissociation constants allowed us to extract ΔGd298(exptl) = 0.3(5) kcal mol–1 from NMR experiments for all-meta tBu-HPE, in good agreement with previous experimental values and B3LYP-D3(BJ)/def2-TZVPP(C-PCM) computations. These computations show a stabilizing trend with substituent size in line with all-meta Ad-HPE (ΔGd298(exptl) = 2.1(6) kcal mol–1) being more stable than its tBu congener. That is, large, rigid, and symmetric hydrocarbon moieties act as excellent dispersion energy donors. Provided a good geometric fit, they are able to stabilize labile molecules such as HPE via strong intramolecular LD interactions, even in solution.
London Dispersion Enables the Shortest Intermolecular Hydrocarbon H•••H Contact. Sören Rösel, Henrik Quanz, Christian Logemann, Jonathan Becker, Estelle Mossou, Laura Cañadillas Delgado, Eike Caldeweyher, Stefan Grimme, and Peter R. Schreiner J. Am. Chem. Soc. 2017, 139, 7428–7431. DOI: 10.1021/jacs.7b01879
Highlights: a) Hydrogens set a short-distance record. Chem. Eng. News 2017, 95 (21), 8; b) Shortest H···H Contact between Hydrocarbon Molecules. ChemViews May 25, 2017. c) Close Encounters of the Hydrogen Kind. JACS Spotlight J. Am. Chem. Soc. 2017, 139, 7665.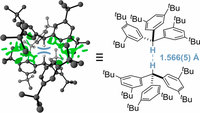
Neutron diffraction of tri(3,5-tert-butylphenyl)methane at 20 K reveals an intermolecular C–H···H–C distance of only 1.566(5) Å, which is the shortest reported to date. The compound crystallizes as a C3-symmetric dimer in an unusual head-to-head fashion. Quantum chemical computations of the solid state at the HSE-3c level of theory reproduce the structure and the close contact well (1.555 Å at 0 K) and emphasize the significance of packing effects; the gas-phase dimer structure at the same level shows a 1.634 Å C–H···H–C distance. Intermolecular London dispersion interactions between contacting tert-butyl substituents surrounding the central contact deliver the decisive energetic contributions to enable this remarkable bonding situation.
Sizing the Role of London Dispersion in the Dissociation of all-meta tert-Butyl Hexaphenylethane. Sören Rösel, Ciro Balestrieri, and Peter R. Schreiner*
Chem. Sci. 2017, 8, 405–410. DOI: 10.1039/C6SC02727JThe structure and dynamics of enigmatic hexa(3,5-di-tert-butylphenyl)ethane was characterized via NMR spectroscopy for the first time. Our variable temperature NMR analysis demonstrates an enthalpy–entropy compensation that results in a vanishingly low dissociation energy (ΔG298d = −1.60(6) kcal mol−1). An in silico study of increasingly larger all-meta alkyl substituted hexaphenylethane derivatives (Me, iPr, tBu, Cy, 1-Ad) reveals a non-intuitive correlation between increased dimer stability with increasing steric crowding. This stabilization originates from London dispersion as expressed through the increasing polarizability of the alkyl substituents. Substitution with conformationally flexible hydrocarbon moieties, e.g., cyclohexyl, introduces large unfavourable entropy contributions. Therefore, using rigid alkyl groups like tert-butyl or adamantyl as dispersion energy donors (DED) is essential to help stabilize extraordinary bonding situations.
Uncovering Key Structural Features of an Enantioselective Peptide-Catalyzed Acylation Utilizing Advanced NMR Techniques. Eliška Procházková, Andreas Kolmer, Julian Ilgen, Mira Schwab, Lukas Kaltschnee, Maic Fredersdorf, Volker Schmidts, Raffael C. Wende, Peter R. Schreiner* and Christina M. Thiele*
Angew. Chem. Int. Ed. 2016, 55, 15754–15759. DOI: 10. 1002/anie.201608559.
Highlight: Frontispiece of communications of this issue.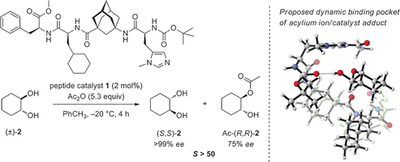
We report on a detailed NMR spectroscopic study of the catalyst-substrate interaction of a highly enantioselective oligopeptide catalyst that is used for the kinetic resolution of trans-cycloalkane-1,2-diols via monoacylation. The extraordinary selectivity has been rationalized by molecular dynamics as well as density functional theory (DFT) computations. Herein we describe the conformational analysis of the organocatalyst studied by a combination of nuclear Overhauser effect (NOE) and residual dipolar coupling (RDC)-based methods that resulted in an ensemble of four final conformers. To corroborate the proposed mechanism, we also investigated the catalyst in mixtures with both trans-cyclohexane-1,2-diol enantiomers separately, using advanced NMR methods such as T1 relaxation time and diffusion-ordered spectroscopy (DOSY) measurements to probe molecular aggregation. We determined intramolecular distance changes within the catalyst after diol addition from quantitative NOE data. Finally, we developed a pure shift EASY ROESY experiment using PSYCHE homodecoupling to directly observe intermolecular NOE contacts between the trans-1,2-diol and the cyclohexyl moiety of the catalyst hidden by spectral overlap in conventional spectra. All experimental NMR data support the results proposed by earlier computations including the proposed key role of dispersion interaction.
London Dispersion Decisively Contributes to the Thermodynamic Stability of Bulky NHC-Coordinated Main Group Compounds. J. Philipp Wagner and Peter R. Schreiner
J. Chem. Theory Comput. 2016, 12, 231–237. DOI: 10.1021/acs.jctc.5b01100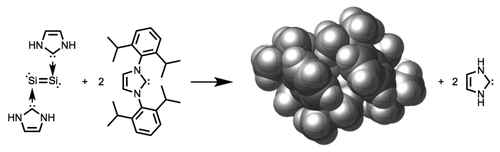
We evaluated the dispersion stabilization of a series of seemingly reactive main group compounds coordinated to bulky N-heterocyclic carbene ligands. We computed the thermochemistry of hypothetical isodesmic exchange reactions of these ligands with their unsubstituted parent systems employing the B3LYP/6-311G(d,p) level of theory with and without dispersion corrections. The energy difference between these two approaches gave dispersion corrections of 30 kcal mol–1 and more. We therefore conclude that London dispersion contributes critically to the thermodynamic stabilities of these compounds. As such, these core–shell structures undergo reactions of the reactive core as long as the dispersion stabilization is conserved.
London Dispersion in Molecular Chemistry–Reconsidering Steric Effects. J. Philipp Wagner and Peter R. Schreiner
Angew. Chem. Int. Ed. 2015, 54, 12274–12296. DOI: 10.1002/anie.201503476. Highlights: a) Frontispiz in the journal; b) oted as very important paper (top 5% of all Angewandte publications); b) Steven Bachrach, Computational Organic Chemistry, January 4, 2016; c) Listed as hot paper (by Thomson Reuters Web of Science) published in the past two years and receiving enough citations to place it in the top 0.1% of all papers in chemistry.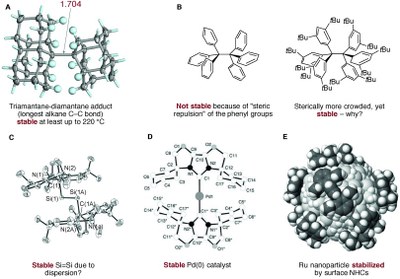
The Self-Association of Graphane is Driven by London Dispersion and Enhanced Orbital Interactions. Changwei Wang, Yirong Mo, J. Philipp Wagner, Peter R. Schreiner, Eluvathingal D. Jemmis, David Danovich, and Sason Shaik
J. Comput. Theory Chem. 2015, 11, 1621–1630. DOI: 10.1021/acs.jctc.5b00075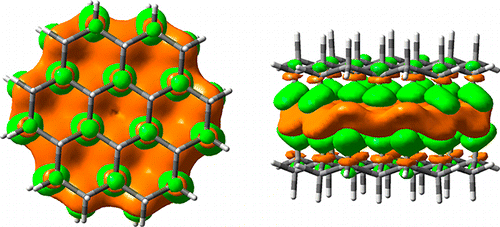
We investigated the nature of the cohesive energy between graphane sheets via multiple CH···HC interactions, using density functional theory (DFT) including dispersion correction (Grimme’s D3 approach) computations of [n]graphane σ dimers (n = 6–73). For comparison, we also evaluated the binding between graphene sheets that display prototypical π/π interactions. The results were analyzed using the block-localized wave function (BLW) method, which is a variant of ab initio valence bond (VB) theory. BLW interprets the intermolecular interactions in terms of frozen interaction energy (ΔEF) composed of electrostatic and Pauli repulsion interactions, polarization (ΔEpol), charge-transfer interaction (ΔECT), and dispersion effects (ΔEdisp). The BLW analysis reveals that the cohesive energy between graphane sheets is dominated by two stabilizing effects, namely intermolecular London dispersion and two-way charge transfer energy due to the σCH → σ*HC interactions. The shift of the electron density around the nonpolar covalent C–H bonds involved in the intermolecular interaction decreases the C–H bond lengths uniformly by 0.001 Å. The ΔECT term, which accounts for ∼15% of the total binding energy, results in the accumulation of electron density in the interface area between two layers. This accumulated electron density thus acts as an electronic “glue” for the graphane layers and constitutes an important driving force in the self-association and stability of graphane under ambient conditions. Similarly, the “double faced adhesive tape” style of charge transfer interactions was also observed among graphene sheets in which it accounts for ∼18% of the total binding energy. The binding energy between graphane sheets is additive and can be expressed as a sum of CH···HC interactions, or as a function of the number of C–H bonds.
