Dr. Katharina Lippert
Research Associate

Field of Work
Morita-Baylis-Hillman reaction
Phase transfer catalysis
Book Review of Asymmetric Phase Transfer Catalysis
Publications:
Structure analysis of substrate catalyst complexes in mixtures with ultrafast two-dimensional infrared spectroscopy. A. T. Messmer, K. M. Lippert, P. R. Schreiner, J. Bredenbeck Phys. Chem. Chem. Phys. 2013, 15, 1509–1517.
Ultrafast Two-Dimensional Infrared Spectroscopy Resolves the Conformational Change of an Evans Auxiliary Induced by Mg(ClO4)2. A. T. Messmer, S. Steinwand, K. M. Lippert, P. R. Schreiner, J. Bredenbeck, J. Org. Chem. 2012, 77, 11091–11095.
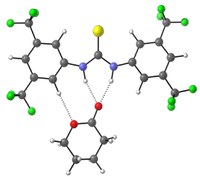 Hydrogen Bonding Thiourea Organocatalysts: The Privileged 3,5-Bis(trifluoromethyl)phenyl Group K. M. Lippert, K. Hof, D. Gerbig, D. Ley, H. Hausmann, S. Guenther, P. R. Schreiner Eur. J. Org. Chem. 2012, 5919—5927.
Hydrogen Bonding Thiourea Organocatalysts: The Privileged 3,5-Bis(trifluoromethyl)phenyl Group K. M. Lippert, K. Hof, D. Gerbig, D. Ley, H. Hausmann, S. Guenther, P. R. Schreiner Eur. J. Org. Chem. 2012, 5919—5927.
Two-dimensional Infrared Spectroscopy Reveals Structural Details of an Evans Auxiliary and its SnCl4 Complex A. T. Messmer, K. M. Lippert, S. Steinwand, E.-B. W. Lerch, K. Hof, D. Ley, D. Gerbig, H. Hausmann, P. R. Schreiner, J. Bredenbeck Chem. Eur. J. 2012, 18, 14989—14995.
(Thio)urea Organocatalyst Equilibrium Acidities in DMSO G. Jakab, C. Tancon, Z. Zhang, K. M. Lippert, P. R. Schreiner P. R. Org. Lett. 2012, 14, 1724—1727.
Hydrogen-Bonding Catalysts: (Thio)urea Catalysis K. Hof, K. M. Lippert, P. R. Schreiner, Science of Synthesis Asymmetric Organocatalysis 2: Brønsted Base and Acid Catalysts, And Additional Topics (Ed.: K. Maruoka), Thieme, Stuttgart, 2011, pp. 297—412.
Cooperative Thiourea–Brønsted Acid Organocatalysis: Enantioselective Cyanosilylation of Aldehydes with TMSCN Z. Zhang, K. M. Lippert, H. Hausmann, M. Kotke, P. R. Schreiner J. Org. Chem. 2011, 76, 9764—9776.
Silicon–(Thio)urea Lewis Acid Catalysis R. Hrdina, C. E. Müller, R. C. Wende, K. M. Lippert, M. Benassi, B. Spengler, P. R. Schreiner J. Am. Chem. Soc. 2011, 133, 7624—7627.
