Gallery of Pictures from 2021
February 2021
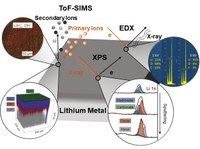
In-Depth Characterization of Lithium Metal SurfacesThe use of lithium metal as anode is an intensively explored option to significantly increase the energy density of batteries. As lithium is highly reactive, its surface is natively covered with a passivation layer that affects the cell performance. However, the passivation layer is mostly not considered and characterized. Therefore, we systematically characterized various lithium samples with X-ray photoelectron spectroscopy (XPS), time-of-flight secondary ion mass spectrometry (ToF-SIMS) and complementary energy-dispersive X-ray spectroscopy (EDX), to give a complete three-dimensional chemical picture of the surface passivation layer. Besides, we explored the factors influencing the passivation layer and the experimental design which is needed to reliably characterize a highly reactive analyte like lithium. (Picture submitted by Svenja Otto.)
View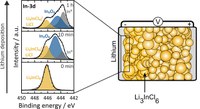
March 2021
Solid-state batteries have been researched and characterized with greater intensity in recent years due to their better properties compared to lithium-ion batteries, such as higher safety or broader operating temperature and comparable ionic conductivities. To compensate for the higher density of solid electrolytes, using lithium metal as anode material is necessary to obtain good gravimetric and volumetric energy densities. However, lithium metal is very reactive. If electronically conductive products are formed during the reaction of the solid electrolyte with lithium, this electrolyte cannot be in direct contact with lithium, otherwise short circuits may occur. In order to investigate the reaction products of the halide solid electrolyte Li3InCl6 with lithium, lithium is applied to the electrolyte by sputter deposition. In situ X-ray photoelectron spectroscopy (XPS) is used to investigate the resulting decomposition products. It was found that Li3InCl6 decomposes into In2O3 and indium metal, among others. Since indium metal is electronically conductive, the electrolyte will decompose until either Li3InCl6 or the lithium is depleted, thus the electrolyte cannot be used in direct contact with lithium. (Picture submitted by Luise Riegger)
View