Gallery of pictures from 2012
Light microscopy image of plasma-oxidized polycrystalline titanium. The colouring depends on different thicknesses of the oxide layer. Picture submitted by Peter Schmitz and Dr. Marcus Rohnke
The figure shows typical cyclic voltammograms of single crystalline platinum electrodes in contact with an aqueous electrolyte (black) and with the oxygen ion conductor yttria-stabilized zirconia (red). The platinum at the interfaces gets oxidized and reduced during the measurement. In case of the aqueous electrolyte in addition the underpotential deposition of hydrogen and the evolution of oxygen are depicted. Picture submitted by Hendrik Pöpke
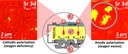
Sr-doped LaMnO3 is the commonly used cathode material for solid oxide fuel cells. During cell operation significant changes of cation surface concentrations occur resulting in crucial effect on the cell performance. The presented experiment focusses on in situ observation of surface composition changes induced by electrochemical polarization applying spatially resolving surface analysis techniques like XPS, SPEM and SIMS and their influence on the cell performance. The figure shows SPEM images of a LSCrM surface after cathodic (left) and anodic polarization (right). The anodically polarized electrode shows small surface areas with enhanced strontium concentrations. During the cathodic polarization the strontium islands disappear and a re-segregation of Sr onto the LSCrM surface occurs with applied anodic bias. The formation of inhibiting SrO surface species under anodic polarization deactivates the electrode whereas the incorporation of the SrO surface layer into the LSM lattice under anodic polarization facilitates the oxygen surface exchange reaction, resulting in an electrochemical activation of the cell. Further information about the electrochemical driven surface segregation processes and their influence on the cell performance of perovskite electrodes for SOFCs offers the following, recently published article: A.-K. Huber, M. Falk, M. Rohnke, B. Luerssen, L. Gregoratti, A. Matteo, J. Janek, In situ study of electrochemical activation and surface segregation of the SOFC electrode material La0.75Sr0.25Cr0.5Mn0.5O3±δ, Phys. Chem. Chem. Phys., 2012, 14, 751–758. (Picture submitted by Anne-Katrin Huber.)
The SEM image shows the cross section of a LiFePO4 thin film on a silicon substrate with a platinum interlayer. LiFePO4 is a promising alternative to the widely-used cobalt-based cathode materials in lithium ion batteries. Experiments on model electrodes can provide new information for a better understanding of the mechanisms of lithium insertion and extraction. The thin films are prepared by pulsed laser deposition (PLD). Film thicknesses typically are between 100 nm and 500 nm, however thinner films are possible if desired. (Picture submitted by Alexander Braun.)
Finite element method (FEM) simulation of the electrical conductivity of a lithium ion conductor with vapor-deposited lithium metal electrodes. The small pictures on the left side show top and down side of the electrochemical cell and the tetrahedral mesh used for the simulation. (red: reference electrode, blue: working electrode, green: counter electrode, black: solid electrolyte) The figure on the right shows the color-coded electrical potential gradient in the sample. Each of the red streamlines represents paths in the electrolyte with an constant current density. (Picture submitted by Dr. Boris Mogwitz.)
In-situ observation of lithium whisker deposition on the surface of a lithium ion-conducting solid electrolyte (pressed and sintered pellet). The electron microscope pictures show a sequence of potentiostatic lithium whisker deposition over a period of 20 h. The enclosed video gives the first 1.5 h in fast motion (28-fold speed). With the aid of a special experimental set-up it is possible to carry out in-situ electrochemical measurements in the scanning electron microscope. Different microelectrodes with tip diameters > 40 nm can be used to contact samples. In this experiment, an Au-microelectrode with a tip diameter of 25 µm was employed as working electrode and macroscopic lithium metal as counter electrode. By cathodic polarization of the microelectrode lithium whisker were deposited on the solid electrolyte and characterized electrochemically afterwards (not shown). (submitted by Rabea Dippel)
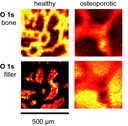
Elemental mapping of bone samples by photoelectron spectroscopy (XPS) The method of photoelectron spectroscopy (XPS) allows the determination of the chemical state of atoms in compounds. By scanning the sample surface with a small-spot x-ray beam it is possible to map the contribution of different atoms and to gain information about their chemical surrounding. The image shows cross sections of vertebral bones of healthy and of osteoporotic rats. The underlying O 1s peak can be attributed to two different chemical binding states which correspond to the bone fibrills and to the embedding material (filler) material, respectively. As brighter points indicate a higher concentration of one component, the loss of bone structure in the osteoporotic sample can be clearly seen. (Picture submitted by Dr. Thomas Leichtweiß.)
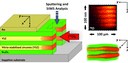
To study the influence of interface structure/strain on the ionic trans¬port, multilayer systems with different number of individual layers (layer thickness: about 22 nm) were prepared by PLD. On the top, a gold capping layer was evaporated to avoid the diffusion of 18O perpendicular to the multilayer system. Then, a slope cut was performed by ion beam milling technique and the 18O-tracer diffusion experiment was performed. Thereafter, the samples were investigated with ToF-SIMS. On the right, 2-D and 3-D elemental mappings of a sample with n = 2 and n = 5 YSZ-layers are presented. In the 2-D mappings bright colours represent a high concentration of 18O. In the 3-D mapping Sc2O3 is green, YSZ yellow and 18O red. Clearly, the 18O only penetrates (starting from the slope) into the individual YSZ-layers. (Picture submitted by Halit Aydin.)
The picture shows a scanning electron microscopy image of the positive electrode in a lithium oxygen battery. During discharge toroidal particles are formed on the electrode surface. (Picture submitted by Conrad Bender and Pascal Hartmann.)
In the figure the scanning electron microscope image of a high voltage cathode material used in lithium ion batteries is shown. To achieve flat thin films, the material was deposited on a platinum-coated YSZ single crystal using pulsed laser deposition (PLD) (YSZ: yttria-stabilized zirconia). As can be seen, no flat films were formed (due to the too high temperature applied in the process), but this rather wavy structure developed which is unfortunately inappropriate for further investigations. (Picture submitted by Mareike Falk.)
Since the end of last year an elementary analyzer (type “vario Micro cube”, Elementar company from Hanau) has been used for chemical analysis of various sample materials in the institute of physical chemistry. The advantages of this instrument are its special specifications like the possibility to analyze very small amounts of sample material below one milligram as well as materials showing high temperature stability like carbon materials or silicon carbides. In addition to the standard measurement of the elements carbon, nitrogen und hydrogen, it is possible to determine the elemental content of sulfur and oxygen, which are of great interest during the investigation of electrode materials for generation four lithium batteries. The picture shows the core of the elementary analysis consisting of the separation column connected to a heat-conductivity detector. The measuring principle is the sensitive and quantitative comprehensible heat-conductivity change of the carrier-gas (helium) caused by addition of the gases CO2, H2O, N2 and SO2, which are formed by combustion of the analyte substances in stoichiometric amounts. (Picture submitted by Christine Raiß.)
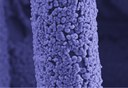
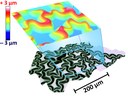
The picture shows a light (bottom) and a confocal (top) microscopic image of a tungsten layer deposited on quartz glass using pulsed laser deposition. The thin layers, produced under vacuum in the PLD chamber, fold after a short time in air as shown in the two images. The contact between the tungsten layer and the quartz substrate is apparently not sufficient after deposition. As substrate and layer cool down the tungsten layer contracts resulting in the observed folds, which are approximately 3 µm high. (Picture submitted by Matthias Kleine-Boymann and Bjoern Luerßen.)