Gallery of Pictures from 2018
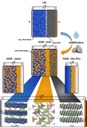
Solidification of batteries from fundamentals to application: In all-solid-state batteries (ASSB) the organic electrolyte is replaced by a lithium ion conducting solid (e.g. Li3PS4), which also act as the separator of cathode and anode. This new cell architecture is presumed to improve the capacity (range of E-cars), power density (charge/discharge time) and safety in the long term. Employees of Prof. Janeks workgroup inquiring fundamental scientific issues, e.g. how lithium ion concentration and changes in the local structure affect lithium ion conductivity in solid electrolytes (doi: 10.1039/c7ta06067j), as well as characterisation and search of novel materials (doi: 10.1021/acs.inorgchem.7b00751), to the investigation of aging mechanisms in running battery cells (doi: 10.1039/C7TA07641J). (Picture submitted by Christian Dietrich.)
Do we always get ZnO as discharge product in electrically rechargeable zinc–air batteries? Nowadays, air breathing batteries appear as a good alternative to overcome our increasing demand of energy. One of the best candidates regarding its cost of production, recyclability and safety is the electrically rechargeable Zn/air battery. Therefore, the development of the rechargeable Zn/air battery has attracted much attention and received an intense research effort to extend its cycling stability. One key issue in alkaline electrically rechargeable Zn/air battery is the electrochemical reaction at the Zn electrode. ZnO as discharge product cannot be fully converted to Zn. In general, the formation of ZnO is influenced by many parameters such as local concentration, pH and diffusion of Zn ions species in alkaline solution. However, the nucleation and growth of ZnO in electrically rechargeable Zn/air battery can be also influenced by an interaction between discharge and charge process. We observed that a battery component, such as a Sn current collector, could also affect the nature of the discharge product during cycling. The scanning electron micrograph (figure below) shows orthorhombic ZnSnO3 or Zn2SnO4 crystallites surrounded by many hexagonal tubes of ZnO obtained after 25 cycles on top of the Zn electrode. These new investigations are part of the ongoing German-Japanese BMBF joint project ‘Zisabi’ to gain a deeper insight into electrically rechargeable Zn/air batteries. (Picture submitted by Saustin Dongmo and Daniel Schroeder.)
When night has fallen over the chemistry building and the scientists have gone home, the research is nowhere near to end. A variety of different electric controllers monitors process parameters as temperature, gas flow, humidity, oxygen activity and many more. By conversion of these analog values into digital signals a connected PC can, thanks to specialized software, monitor and control the measurement process at any time – regardless if those are measurements of functional solid oxides with slow kinetics or of novel battery cells which are analyzed over hundreds of charge/discharge cycles. (Picture submitted by Jonas Neumeier.)
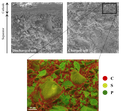
Lithium-ion-batteries are used in many fields of our daily live due to their high energy- and power density. Usually, graphite anodes and transition metal cathodes are used as electrodes. LiNi1/3Co1/3Mn1/3O2 (NCM) particles are often used as cathode material. Increasing nickel content of the material results in higher capacities at the expense of stability. Doping or coating of the material can mitigate this effect. Time of flight secondary ion mass spectrometry (ToF-SIMS) enables to detect even very low elemental concentrations. This method is applied to investigate whether dopants are incorporated into the material or deposited at the particle surface. The upper image shows a mass image as RGB overlay of the surface of a NCM electrode. A focused ion beam (FIB) was applied to produce a cross section. The NCM particles (oxygen red; lithium blue) are embedded into a carbon matrix (carbon green). This makes it possible to investigate the composition of the surface and the core of the particles. The lower image shows a 3D reconstruction of the oxygen signal, which was measured during a FIB-SIMS-tomography of the sample. (Picture submitted by Jan Binder and Marcus Rohnke.)
Eight thousand two hundred and fifty – about this number of cylindrical 18650 lithium-ion cells are required to produce a 100 kWh battery module. With good batteries, a fully charged 100 kWh battery module can power the car over a distance of more than 500 km. In the event of an accident or faulty cells, it is possible that the battery overheats due to an internal short circuit and ignites the liquid electrolyte contained inside the cells. The 18650 cells contain about 10 wt.% of liquid electrolyte. At a weight of about 48 g per cell, the 100 kWh battery module contains in total about 40 kg of flammable liquid electrolyte. This corresponds approximately to the amount of gasoline in a full fuel tank of a car with internal combustion engine, thus posing a safety risk in case of an accident.In solid-state batteries, a solid electrolyte is used as ion conductor instead of the liquid electrolyte. Thus, the entire cell is built up of solids, which significantly reduces the risk of self-ignition of the battery. In addition, solid electrolytes offer a research approach to allow use of lithium metal as anode material, so that energy density of the cells can be increased. Due to lithium dendrite growth and the resulting short circuit of the lithium-ion battery, lithium metal cannot be used as the anode material in combination with a liquid electrolyte. In the project FELZIA (Festelektrolyte als Enabler für Lithium-Zellen in Automobilen Anwendungen), funded by the Bundesministerium für Bildung und Forschung, we explore solid-state batteries with lithium metal in cooperation with partners from industry and other academic research institutions. The goal is to develop solid-state batteries with high energy and power density. (Picture submitted by Felix Richter)
Classical boundary scattering in semiconducting InAs nanowires: Semiconducting nanowires are of great importance as nano-devices for future electronic and optoelectronic applications, such as e.g. field-effect transistors, gas sensors or light-emitting diodes. Such semiconductor nanowires are typically one micrometer in length and have a diameter in the range of less than one hundred nanometer. On these small dimensions, the nanowires also show interesting quantum-mechanical transport phenomena. The figure shows the change of the electrical resistance of a single InAs nanowire in an applied external magnetic field for an orientation of the field parallel and perpendicular to the axis. Typically, semiconductors exhibit a quadratic magnetoresistance, whereby the resistance should be linear with the square of the field. This is indeed the case for a parallel orientation of the field to the wire. However, if the external field is oriented perpendicular to the nanowire, the resistance exhibits a kink. This behavior indicates additional scattering of the electrons at the interfaces of the nanowire. Due to the field perpendicular to the direction of the current the electrons are deflected by the Lorentz force on circular paths. In the case of small fields, the electrons scatter at the interface (classical interface scattering) resulting in an additional contribution to the magnetoresistance (region I). Increasing the field reduces the radius of the circular path due to the stronger Lorentz force and increases the probability of backscattering. Above a certain critical magnetic field strength, the radius of the circular path becomes smaller than the diameter of the wire and the contribution of the interface scattering decreases (region II), which leads to the kink in the magnetic resistance. (Picture submitted by Patrick Uredat and Matthias Elm.)
The two pictures show the chamber inside of our new Xe plasma FIB SEM (XEIA3, Tescan) and the preparation process for the cross section of a zinc sponge which incorporates an ionomer. At first, a trench was made with the highest milling rate in about 2 hours, followed by polishing with two successive steps of an intermediated and a low milling rate for about 1 hour. The cross section shown is the first cross section made in our new FIB SEM. The sample was investigated within Project Zisabi (in cooperation with Prof. Abe, Kyoto University, Japan; funded by the German Federal Ministry of Education and Research), where we look at the influence of anion-exchange ionomers on structured zinc anodes to stabilize the electrochemical deposition and dissolution processes during cycling.For more information, please contact Boris Mogwitz and Klaus Peppler. Publication on this topic:Stock, D. et al. Towards zinc-oxygen batteries with enhanced cycling stability: The benefit of anion-exchange ionomer for zinc sponge anodes. J. Power Sources 395, 195–204 (2018).(Picture submitted by Daniel Stock, Boris Mogwitz and Klaus Peppler.)
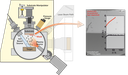
The crucial part of battery engineering is interface engineering, especially when it comes to lithium metal anodes. An ideal interface prevents the electrolyte from reacting with lithium without impairing the battery performance. One possibility for the formation of optimized interfaces is the application of a protective layer between the electrolyte and the lithium anode.A suitable method to deposit such layers is ion beam sputtering. The controlled sputtering rate leads to very thin but uniform layers. In combination with an additional plasma source (cf. schematic) an oxidation or nitridation of the protection layer is possible. This method enables the application of a multitude of materials. Already a 1 nm - 5 nm thick interlayer is able to significantly slow down the increase of the total resistance Rt of the battery (cf. comparative measurement on the right part of the picture). (Picture submitted by Matthias Geiß).
Owing to the high theoretical gravimetric energy density based on the conversion reaction between S and Li2S, Li-S batteries have attracted attention as a promising candidate for the next-generation energy storage system. Although the notorious shuttle effect, deteriorating the battery capacity due to the formation of polysulfides soluble to the conventional electrolytes, can be physically hindered by employing solid electrolytes (SEs), the mechanically rigid property of inorganic materials causes another crucial issue: the chemo-mechanical failure. The liquid electrolyte maintains good contacts to active materials by infiltrating the gaps and cracks formed during the lithiation/delithiation. However, while Li2S shrinks significantly when it is converted to S by charging, the contacts between components can be lost as seen in the SEM cross-section image of the charged cell, which leads to the capacity loss. Cathode composites shown here contain active material S, conductive carbon additives, and the SE containing P. A deeper understanding of the failure mechanisms is key to the further development of solid-state Li-S batteries. (Picture submitted by Saneyuki Ohno.)
In order to develop safe and efficient solid state batteries, solid electrolytes with high ionic conductivity must be developed. It was found that the lattice dynamics of crystalline solid electrolytes, i.e. the dynamic oscillation of the lattice atoms, correlates strongly with the transport of the mobile ions in the solid. The picture shows the structure of a thiophosphatic sodium ion conductor whose sulfur anions (reddish) can be substituted with selenium (yellowish). With the exchange of sulfur with the bigger and more polarizable selenium, a lower activation barrier for an ion jump from one lattice site to an adjacent site could be observed experimentally. This is shown schematically on a flattened 3D potential landscape. In parallel, a frequency minimization of the lattice oscillations (phonon DOS) can be observed, which can be measured experimentally by inelastic neutron scattering, but also predicted theoretically. This frequency minimization goes hand in hand with the observed trends in ionic conductivity and shows that for the screening of possible high-performance solid ion conductors the lattice dynamics as a descriptor must be investigated and understood in more detail. (Picture submitted by Thorben Krauskopf.)
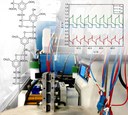
Redox flow batteries are suitable for the stationary storage of intermittent energy provided by renewable energies. The beneficial design of the redox flow battery enables the storage of energy in liquid electrolytes in different oxidation states outside the actual electrochemical cell. Currently, the active material dissolved in the electrolyte constitutes the main cost factor for redox flow batteries. Using cheaper organic molecules instead, that for example may be obtained by purification of waste products from the pulp and paper industry, could save costs and further increase the economic viability of redox flow batteries. In the search for a suitable molecule that fits this application, the BMEL project FOREST is currently investigating several organic molecules for their stability and performance. The picture shows the measurement setup used for a lab-sized redox flow battery (center). The dissolved active materials – here in the form of an organic electrolyte on the anode and a vanadium (IV) based electrolyte on the cathode side – are transported into the electrochemical cell by pumps. A reference electrode allows for the recording of the working (WE) and counter electrode (CE) potentials during battery cycling and therefore enables to draw conclusions about the performance and degradation behavior of the associated active materials (right). Picture submitted by Dominik Emmel and Jonas Hofmann, RG Dr. Daniel Schröder
To resolve the conductivity of both individual grains and grain boundaries within a ceria (CeO2) thin-film, gold microelectrodes were deposited in such a way that only a minimal area of the grains would be exposed to the gold, thereby preventing unwanted conduction pathways throughout the thin-film. To accomplish this, an insulating layer of alumina (Al2O3) was deposited on top of the thin-film in order to cover everything except the desired contact areas on the grains. For this and for the preparation of the electrodes, several iterations of photo and electron beam lithography were employed. At first, the fine structures at the grains were transferred, followed by the deposition of the contact pads on the edge of the thin-film, which were needed to connect the sample to the measurement setup.With these electrodes, it was possible to perform impedance spectroscopy on both individual grains and grain boundaries at temperatures up to 773 K, while varying the oxygen partial pressure in order to gain insight into the differences in defect chemistry arising from space charge layer effects. (Picture submitted by Julian Zahnow.)