Gallery of Pictures from 2019
January 2019
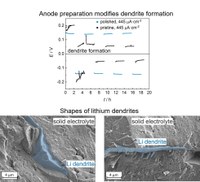
A major problem of current battery technology applying liquid, organic electrolytes is the formation of lithium dendrites. When they reach the cathode side, this results in a short circuit of the battery module. Leading to the module failure, up to the formation of toxic and combustible gases. Dendrites form due to nonuniform deposition of lithium on the anode side during battery charging. Liquid electrolytes and polymers cannot prevent dendrite formation within the electrolyte. Therefore, solid, inorganic and crystalline electrolytes have become focus of research, as they should not only have high conductivity, weight and volume savings (higher energy densities) and significantly lower temperature dependence of conductivity, but also sufficient density and a high shear modulus to effectively prevent the growth of dendrites.However, recent research in AG Janek has shown that it is still possible for lithium dendrites to grow through solid electrolytes, which are relevant for industrial applications. Critical factors for the formation or absence of dendrites are amongst others the temperature, the applied pressures, the applied current densities, and the preparation of the lithium metal foils (anode) used. The exact influences and critical parameters of these factors must be further investigated and the mechanism of dendritic formation in solid state batteries must be clarified and understood.In order to achieve an effective inhibition of dendrite formation, it is possible to synthesize novel solid electrolytes or to modify and protect the interface between anode and solid electrolyte with novel compounds. (Picture submitted by Simon Randau.)
View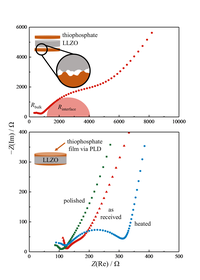
February 2019
Driven by the interest in high-performing and safe solutions for energy storage, continuous progress was made in the field of all-solid-state lithium batteries over the past years. Although modern thiophosphate electrolytes provide ion conductivities comparable to the widely used liquid materials, their low stability against lithium is still a major bottleneck for application. However, less conductive oxide electrolytes, e.g. Li7La3Zr2O12 (LLZO), show superior stability, especially in contact with metallic lithium, which is a targeted anode material in all-solid-state cells.While the symbiotic combination of materials to composites is used for a variety of purposes, composite electrolytes represent a comparably unexplored field in research. By combining different compounds, the individual benefits may lead to new possibilities for cell design. As ionic transport is naturally the key performance parameter for electrolytes, the interfacial resistance was investigated in a first step. While simply pressing solids only leads to insufficient contact (upper figure), pulsed laser deposition was employed to ensure an intimate junction between the materials. In this work, the influence of surface treatment of a LLZO pellet on the resulting interfacial resistance towards a thiophosphate electrolyte was investigated. LLZO is known to form a passivating layer on its surface, which suppresses charge transfer. By impedance analysis, the contribution of the interface can be resolved. Polishing of the surface leads to an interfacial resistance as low as 7 Ω∙cm2 at room temperature, while heating the pellet increases the interfacial barrier. Besides a more detailed analysis on the developing interface, a bilayer electrolyte will be implemented into an all-solid-state cell as a next step. (Picture submitted by Georg Dewald.)
View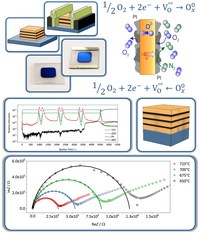
March 2019
Oxygen production is of paramount importance for steel and chemical industry. Nowadays it is widely carried out in large scale using cryogenic distillation processes, which need to be volume and energy intensive in order to be profitable and viable. An average oxygen production plant produces 4000 to 10000 tons/day using 200 kWh/tons. The implementation of oxide conductors opened the way for oxygen permeation ceramic materials for more energy sustainable processes. The basic requirements are high electronic and ionic conductivities, thermal and chemical stability. The main preparation route for these materials is using high amounts of noble metals such as Pd or Pt (up to 50% V/V) to grant the needed electronic conductivity and this makes the large-scale application economically not viable. A possible solution could be to minimize the amount of the electron conducting material in nanostructured composite materials. Several nano-structured porous YSZ thin films homogeneously coated with 12 nm of Pt using atomic layer deposition were prepared. In order to assess the interface contribution on the defect concentration and the catalytic effect of the Pt presence on the oxygen redox reactions, a series of multilayered YSZ-Pt-YSZ samples were prepared. The as prepared materials were structurally characterized using grazing incidence X-ray diffraction (GIXRD) and time of flight secondary ions mass spectrometry (TOF-SIMS). The polycrystalline structures of both Pt and YSZ were confirmed as well as the expected nanostructure. Electrochemical impedance spectroscopy (EIS) measurements are performed to correlate the structural and morphologic features of the materials with the ionic transport and in particular to elucidate the influence of the interfaces on the transport properties. (Picture submitted by Michele Bastianello.)
View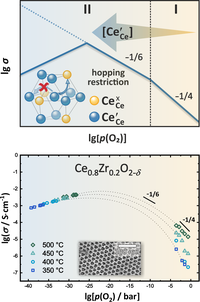
April 2019
Ceria is a redox-active oxide with a high tendency to form point defects. Under reducing conditions the material releases oxygen, forming an oxygen vacancy and preserving electroneutrality by the reduction of two tetravalent cations Ce4+ (CeCe×) to Ce3+ (CeCe′). These localized electrons can then undergo an activated hopping mechanism and contribute to electrical conductivity as small polarons. The Brouwer diagram predicts the increase of charge carrier concentration with decreasing oxygen partial pressure and is a popular tool to explain the conductivity behaviour of nonstoichiometric oxides by assuming constant defect formation enthalpies. Instead of an increase in conductivity with decreasing p(O2) mesoporous Ce0.8Zr0.2O2-δ thin films reveal a decrease in conductivity under strongly reducing conditions, which cannot be explained by standard Brouwer assumptions. The experimental results can be understood by including elementary statistics in the hopping process of the electron polarons. When the concentration of Ce3+ exceeds the one of Ce4+, the corresponding energy level for the small polaron at possible hopping sites is already occupied and hopping is therefore restricted. The electrical conductivity decreases, which has not been observed for polarons in ceria so far and can only be explained by a combination of enhanced reducibility and high surface area of the mesoporous samples. (Picture submitted by Kathrin Michel.)
View
May 2019
View
June 2019
The last year's Christmas lecture of the Department of Chemistry had the topic "Water - The elixir of life and its chemistry". The picture shows an impressive example of the power of water: A bottle of colored cooking oil is gently lowered into a cylinder filled with water. Although the oil has a lower density than water, it is held in the bottle due to the larger surface tension of water (γ (H2O) = 0.073 N∙m−1 compared to (γ (oleic acid) ≈ 0,033 N∙m−1). Not before a drop of washing-up liquid is added, the oil flow upwards. (Photos by Heiko Barth (Institute for Didactics of Chemistry) and Bjoern Luerßen (Institute of Physical Chemistry), picture submitted by Bjoern Luerßen.)
View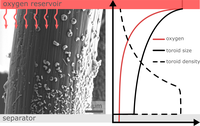
July 2019
Rechargeable metal-oxygen batteries are considered as a potential technology in future energy storage systems. Alkali metal-oxygen batteries, such as lithium-oxygen batteries, are in particular focus of industry and research due to their high theoretical energy density. The lithium peroxide formed during discharge precipitates as a solid on the cathode structure, so the cathode design and understanding of the growth mechanism play a crucial role in achieving maximum energy densities. For this reason, the BMBF-project MeLuBatt – in close cooperation with the Institute for Energy and Systems Engineering (InES) at TU Braunschweig and the Fraunhofer Institute for Manufacturing Technology and Applied Materials Research (IFAM) in Oldenburg – is currently investigating the growth behaviour of the discharge product in the cathode of lithium oxygen batteries. The SEM image shows the magnification of a carbon fiber on which toroid-like Li2O2 particles have been formed. The size and particle density of these toroids strongly depends on the availability of dissolved oxygen in the electrolyte: With more dissolved oxygen (cathode side facing the O2 reservoir), fewer but significantly larger toroids are formed, while deeper in the electrolyte (cathode facing the separator side) the toroids are smaller, but grow in a higher density. The diagram schematically illustrates how the size and density of the toroids depend on the dissolved O2 in the electrolyte along a carbon fiber. (Picture submitted by Julian Kreißl, Daniel Langsdorf and Daniel Schröder).
View
August 2019
Atomic Force Microscopy (AFM) is a method of characterizing a sample surface by passing a tip close to the surface to be examined. By measuring the atomic forces between the tip and the surface by means of the deflection of the tip, it is possible to obtain information about the topography of the surface or to determine the magnetic and chemical properties of the surface. The picture on the left shows the topography of a cathode for lithium-ion batteries investigated by AFM, in which as active material secondary particles of Li(Ni,Co,Mn)O2 with a diameter of a few micrometers are embedded in carbon. In the right image, an electrically conductive tip was used during the AFM measurement to examine the electrical conductivity of the cathode at the surface. Clearly recognizable is the impact of carbon as conductive additive, since an electrical current is measured mainly in the region, where carbon is found. (Picture submitted by Miguel Wiche and Matthias Elm)
View