Gallery of Pictures from 2017
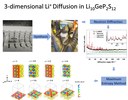
3-dimensional diffusion pathways in a solid ion conductor -Besides the establishment of novel preparation and processing methods, the development of functioning solid state lithium batteries requires deep understanding of the underlying intercalation- and diffusion mechanisms in the used components. Li10GeP2S12 (LGPS) is one of the most popular solid lithium ion conductors due to its enormously high ionic conductivity of more than 10 mS/cm. The picture shows the different steps from the synthesis of the light gray compound to the visualization of lithium diffusion pathways, which were determined from neutron powder diffraction, utilizing the maximum-entropy-method (MEM). This method allows to illustrate the actual distribution of the nuclear density for lithium, which has a negative scattering length in neutron diffraction. It becomes obvious that the tetragonal elementary cell of LGPS does not only comprise diffusion pathways for lithium ions along the c-axis, but also in the a-b-plane. (Picture submitted by Dominik Weber.)
For the development of stationary energy storage hydrogen is an environmental way to store energy in form of chemical energy. Therefore, the reaction at the anode is of great interest. A fundamental problem is the large overpotential and the stability of the electrode to produce oxygen at low costs. These essential conditions are only fulfilled by rare earth metal oxides which are expensive to purchase. Nature uses a calcium-manganese oxide complex to catalyze the oxygen development reaction. The mechanism behind is not fully clarified, which makes these material class an interesting field of research. In the picture an activity and stability measurement of a manganese catalyst is shown. Aim of the research is to influence the defect chemistry of binary and ternary manganese oxides to improve the activity and stability of the crystal phase. (Picture submitted by Raika Oppermann and Christoph Richter.)
Lithium-ion-batteries (LIBs) have become indispensable in many areas in our lives. LIBs exhibit both high energy density and high power density, which make them particularly suitable for electric vehicle applications. Regarding the battery components, LiNi1-x-yCoxMnyO2 (NCM) is frequently employed as the cathode active material. Interestingly, it was recently proposed that the nickel ions could be reduced to the divalent state when the Li ions are being shuttled between the electrodes during cycling. Given that this effect would hinder the electrochemical performance, it is therefore important to gain a better understanding of such mechanisms occurring during battery operation. By using synchrotron X-ray diffraction, it is possible to obtain very high-resolution structural data (e.g., pair distribution functions), which provide very precise information about the local crystal structure. Once the local structure is elucidated, the underlying mechanisms for the cation mixing become clearer, thereby enabling new development strategies toward mitigating the associated electrochemical issues. The figure shows an exemplary pair distribution function of NCM and two of the representative probability densities for atomic distances within the unit cell. (Picture submitted by Jan Binder.)
Due to their high oxygen storage capacity, ceria zirconia solid solutions (CexZr1-xO2) are frequently used in catalytic applications, such as the three-way catalytic exhaust gas converter. Recently, nanostructured ceria-zirconia-based materials also attracted much attention due to enhanced proton conductivity at low temperatures, whose origin is still under debate.To study the influence of humidity on the electrical transport processes, we use a model system based on ceria zirconia thin films prepared by pulsed laser deposition. In order to measure the conductivity of the thin films parallel to the surface, platinum interdigital electrodes (light grey structure) were prepared by photolithographic methods in the Micro- and Nanofabrication Lab (MiNa) at the Center for Materials Research. The image at the left shows the temperature-dependence of the total conductivity obtained from impedance measurements on a ceria zirconia thin film shown in the SEM image on the right. At low relative humidity, an enhancement of the total conductivity is observed only at low temperatures due to proton conductivity. At high relative humidity, a conductivity plateau forms within the temperature range between 400 °C and 30°C arising due to the formation of a water layer on the thin films. (Picture submitted by Matthias Kleine-Boymann)
The crystallization of a non-stoichiometric and amorphous thin film is investigated by transmission electron microscopy at elevated temperatures for comparing the results with X-ray diffraction data. The in-situ TEM pictures at 75 °C of an initially amorphous zirconium oxide thin film with a formal composition 1:1 (Zr:O) are shown. The nucleation proceeds already at lower temperatures at the platinum interface and the two grains (50 nm x 30 nm) expand and grow together within a few minutes.Further information (e.g. diffraction pattern) on the properties of the thin film material “ZrOx” can be found in: Phase formation and stability in TiOx and ZrOx thin films: Extremely sub-stoichiometric functional oxides for electrical and TCO applications, Z. Kristallogr. 2017; 232(1-3): 161-183, DOI: 10.1515/zkri-2016-1981, De Gruyter(Picture submitted by Ralph Henning.)
Scanning electron micrograph of a cathode composite of Li[Ni,Co,Mn]O2 and Li3PS4 as solid electrolyte, prepared in a bulk-type solid-state cell. After cycling, the pelletized battery cell was disassembled in an inert gas atmosphere. A nanometer-sized spherical gap was formed around the secondary particles of the active material after electrochemical treatment. NCM particles contract during de-lithiation (charge) and lose contact with the solid electrolyte. The result is a decreased capacity due to partially isolation of particles. (Picture submitted by Raimund Koerver.)
The picture shows X-ray photo electron spectroscopy (XPS) detail spectra of the elements oxygen, carbon and lithium observed on a Lithium surface which was in contact to the battery electrolyte 1.3-dioxolane. Organic solvents decompose on metallic lithium leading to a passivation of the surface. Using XPS we could identify these decomposition products by detecting elements and distinguish their oxidation states. The challenge is to identify the resulting signal pattern of each component represented in the picture by the line structure above the spectra. (Picture submitted by Carsten Fiedler.)
Determination of the mobility of Sr2+ in cortical bone. Within the framework of SFB TRR79, new replacement materials with drug depots are being developed in order to improve fracture healing after an osteoporotic fracture. Those active species are released after implantation over a period as long as possible and to stimulate bone formation locally. Strontium has a dual effect on bone healing: bone-building cells (osteoblasts) are stimulated and bone-degrading cells (osteoclasts) are inhibited. The complete elucidation of the transport of active substances such as Sr2+ in bone can help to predict and simulate the drug mobility in bone and thus reduce animal experiments.a) ToF-SIMS. The upper pictures show the images of Ca2+ (mineralized bone) and Sr2+ signals. In the lower images, the spatial distribution of Ca2+ and Sr2+ in cortical bone is shown in a 3D rendering (depth profile). b) Confocal microscope. Image of the crater in the cortical bone caused by sputtering. (Picture submitted by Christine Kern)
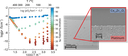
The electrochemical synthesis from Ionic Liquids (ILs) offers an alternative route for the formation of unusual noble metal compounds, like silver oxides. A major advantage of this route is the possibility to work in the absence of harsh conditions like high pressures or temperatures and the use of highly reactive and/or hazardous educts. This approach is analogous to the reactions in a metal air battery, where oxygen is reduced to superoxide at the cathode (Red1: O2 + e– → O2–) and metal oxidized to the corresponding metal ions (Ox, here: Ag → Ag+ + e–). These can precipitate together as a metal oxide (here: AgxOy) and/or undergo further disproportionation. However, the metal ions compete with the oxygen of the reduction at the cathode and the inverse reaction to Ox can occur (Red2: Ag+ + e– → Ag) instead of the wanted oxygen reduction reaction (ORR).A pure oxygen saturated IL ([Pyr13][TFSI]) shows a clear ORR signal (Red1) in the cyclic voltammogram at -1.17 V (left figure, blue line). After the addition of the corresponding silver salt (Ag[TFSI]) the peak vanishes and another redox potential at -0.38 V appears, which corresponds to the Ag/Ag+ potential. Thus, unlike in metal air cells, no reaction of oxygen and silver to the metal oxide (Red1 and Ox) is observable. Instead, pure silver is deposited on the cathode and the ORR is suppressed (Red2 and Ox). The right figure shows the porous morphology of the deposited silver.The major target of this project is a better understanding of the occurring reduction reactions Red1 and Red2 depending on parameters like temperature, scan rate, potential, O2- and Ag-concentration. (Picture submitted by Peter Schmitz)
In order to explore fundamental effects of lithium ion batteries it is typically important to prepare model systems in order to study individual effects on a given system. The preparation and analysis of thin and planar films of electrode materials for example can allow insights into the interface interaction between these materials and the electrolyte. The preparation of these films is influenced by a multitude of different parameters. In the given example the influence of the substrate material on the film morphology and texture is depicted.The thin films shown were prepared by spin coating of LiNi0.5Mn1.5O4 on silicon wafers and yttria doped zirconia (YSZ/Pt). In this method a stoichiometric solution of the corresponding metal salts is dripped onto a spinning substrate and the resulting film is crystalized in an oven. The thickness of the film can be controlled by depositing multiple layers. The samples on silicon (a) and a cross section c)) show very poor adhesion and are strained which causes cracks and deformations to form. On YSZ/Pt however (b) and cross section d)) an identically prepared film appears stable and individually deposited layers are visible in the cross section. Therefore, the preparation conditions have to be controlled specifically for every substrate material. (Picture submitted by Patrick Schichtel.)
To enable large industrial application of next generation batteries several problems have to be solved. In the case of all-solid-state batteries such problems are for example a bad cycling stability and capacity fading. In this context, a deeper understanding of the degradation mechanisms is necessary to improve the battery performance. Investigations regarding the interface between the cathode and the electrolyte are typically based on electrochemical studies, diffraction methods, microscopic investigations and computational methods. In contrast, the measuring possibilities of analytical methods such as time-of-flight secondary ion mass spectrometry (ToF-SIMS) are yet not fully exploited.The image shows 3D tomography studies of uncoated and coated Li[NixCoyMnz]O2 (x + y + z = 1) particles which were performed with ToF-SIMS. Ideally, coating the cathode material should lead to an improved battery performance and the coating should be homogeneously distributed on the surface. The image shows clearly that ToF-SIMS is a valuable tool for investigations regarding the coating of battery materials. The signals of the particles can be easily distinguished from the coating signals, which allows a characterization of the coating morphology. ToF-SIMS provides structural and chemical information simultaneously with a high lateral resolution (~ 100 nm) which is not easily accessible through other techniques. (Picture submitted by Felix Walther.)