Gallery of pictures from 2014
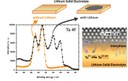
The picture shows the X-ray photoelectron spectra (Ta 4f) of a lithium ion conductor without and with a sputtered lithium layer. Since approximately six months this type of in situ experiments can be performed in our group. Here, it can be clearly seen that the Tantalum ion is reduced if the solid electrolyte is covered with Lithium (grey spectrum). With ongoing time, this reduction zone proceeds through the whole volume of the electrolyte and thus, the electronic conductivity is increased caused by partially or totally reduced Tantalum (small sketch down right). (Picture submitted by Sebastian Wenzel.)
The large picture shows the recipient of our newly obtained sputter deposition system. The generated plasma can be seen in the small picture. Since this sputter deposition system has a direct connection to a glove box, all samples can be handled in an argon environment without contact to atmosphere. This addition to the physical vapor deposition (PVD) methods enables the research group of Prof. Janek to deposit large, homogeneous thin films using 4 inch targets. (The deposition system was acquired through the financial support of the BASF SE, Ludwigshafen. Picture submitted by Jochen Reinacher.)
Transition metal oxides and especially CeO2 are known for their ability to exchange oxygen with the surrounding gas atmosphere. This behaviour has significant impact on the electrical conductivity, and with decreasing p(O2) oxygen vacancies and electrons are formend by loss of oxygen. An alternative class of oxygen storage materials, namely mayenite (Ca12Al14O33, abbrev. C12A7:O), shows a cage structure formed by CaO and Al2O3 (see inset). Between these cages, oxygen ions are highly mobile at elevated temperature. The conductivity of this material is also affected by hydration, which depends on p(H2O) (humidity). The hydration of mayenite can be expressed as follows: Ca12Al14O33 + H2O → Ca12Al14O32(OH)2 At very high humidities the conductivity follows the relation lg σ ∝ lg p(H2O)^-1 whereas at low humidities it comes close to the pure oxygen ion conductivity. In terms of a defect chemical model the course of the conductivity vs. p(H2O) can be well explained. (Picture submitted by Jens-Peter Eufinger.)
Ionic liquid based electrolytes (IL electrolytes) show promising properties with respect to their application in batteries. However, the lithium ion conductivities in these electrolytes are low. By adding silica as filler materials the properties of the IL electrolytes are supposed to be improved due to an interface effect. As shown in the figure above the conductivity behavior in dependence on the amount of filler material cannot be explained simply by the viscosity change since the composite electrolyte with the higher viscosity increase shows also the higher conductivity. Thus, it can be assumed that ion conductivity of a certain ion species is increased by the interface effect. (Picture submitted by Nastaran Krawczyk.)
KaleidoscopeHigh lateral resolution ToF-SIMS mass image received during the analysis of an implanted biomaterial (modified Calciumphosphat) within the transregional collaborative research centre SFB/TRR 79. The image shows the interface of bone and bone marrow as an overlay of the Calcium signal (green), the amino acid fragment C4H8N+ (blue, representing the collagen) and the sodium signal (red). The image is mirrored vertically and horizontally. (Original size is 99 x 99 μm2 with a lateral resolution of 300 nm.) (Picture submitted by Dr. Anja Henß.)
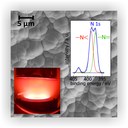
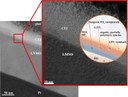
The picture shows a scanning electron microscopy image of a dense, lithium ion conducting thin film of amorphous lithium phosphorous oxinitride (“LiPON”). It has been deposited on a porous substrate via RF-magnetron-sputtering of a lithium phosphate (Li3PO4) target in a nitrogen atmosphere (bottom left). The nitrogen incorporates throughout two different coordination numbers that can be identified via x-ray photoelectron spectroscopy (XPS, top right). The films of thicknesses between several hundred nanometers and a few microns achieve overall conductivities in the order of 10−6 S∙cm−1. (Picture submitted by Martin Busche.)
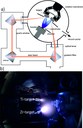
In figure a) one can see a drawing of a dual beam pulsed laser deposition chamber in our group. At the entry a KrF-Laser (248 nm) is splitted into two beams using a gradient filter (semitransparent mirror). The beams are directed and focused with secondary mirrors and lenses on rotating targets (here: zirconium and titanium). Additionally, one can heat the substrate and the ablation process can be performed in different gas atmospheres. In figure b) one can see a picture while ablating titanium and zirconium in an oxygen atmosphere. One can see the different sizes of the plasmas, which correspond to a higher energy (60 % of the total energy) on the zirconium target compared to titanium (40 %). The positioning of the gradient filter determines the ratio of the energies. (Picture submitted by Ralph Henning.)
The picture shows a scanning electron microscopy image of a ceria (CeO2) thin film, deposited by pulsed laser deposition (PLD) on a sapphire (Al2O3) single crystal substrate. An interdigital platinum electrode, structured by photolithography, contacts the ceria thin film. The picture shows a cross section through the different layers.We use these layer systems to determine the conductivity of the mixed conductor ceria at different temperatures and gas atmospheres using electrochemical impedance spectroscopy. From these data, we can draw conclusions about the transport properties of the ceria thin films. As it is possible to prepare thin films with a well-defined morphology, this model system allows us to investigate the influence of the morphology on the transport properties of CeO2, representative for a wide variety of polycrystalline, mixed conductors. (Picture submitted by Matthias Kleine-Boymann.)
Transmission electron microscope (TEM) image of a cycled LiNi0.5Mn1.5O4 thin film electrode, showing the cathode electrolyte interphase (CEI) on its surface. This interface film is composed of organic as well as inorganic decomposition products of the electrolyte, the electrolyte salt as well as of reaction products between the electrolyte and the cathode. It shows a stacked microstructure. Rather inorganic species are located close to the electrode, whereas more organic ones are situated near the electrolyte.The thin film electrode was deposited by pulsed laser deposition onto a platinum covered YSZ single crystal. The TEM images were recorded at the Institute of Materials Physics, University of Münster. Thanks to Y. Hamedi and Dr. F. Berkemeier for these measurements. (Picture submitted by Dr. Mareike Falk.)
The lithium-sulfur battery currently attracts worldwide attention due to its high energy density and the low cost components. A central aspect of current research is the preparation of special sulfur/carbon composite materials for the positive electrode that ought to improve the cycle life. Usually, the characterization of these nanostructured composites is done by vacuum-based methods, mainly by nitrogen physisorption and scanning electron microscopy (SEM). However, we could show by systematic analyses of some sulfur/porous carbon model composites that the obtained results from these methods can be misinterpreted easily. For nitrogen physisorption for example the expected result would be that identical isotherms were found for the pure carbons and their mixed composites with sulfur, since sulfur itself is non-porous and the results are normalized on the amount of the porous carbon. Yet the figure shows that rather an extreme loss of porosity was observed for the mixed composite compared to the pure carbon. In situ SEM studies with energy-dispersive X-ray spectroscopy (EDS) could prove that an unexpected, rapid sulfur redistribution with remarkable extent takes place in the carbon material during this measurements. For example 13 wt% sulfur could be detected in the originally sulfur-free carbon material after three hours at SEM vacuum, which also give an explanation for the observed physisorption results. This shows that characterizing the structure of sulfur/carbon composites or electrodes is very challenging and the observed rapid sulfur migration might also have important consequences for the performance of lithium-sulfur batteries.(Picture submitted by Christine Eufinger)
Fig. A) shows a scanning electron microscopy image of a sodium superoxide crystal, grown on a carbon fiber of the positive electrode of a sodium oxygen battery during discharge. Fig. B) shows the x-ray diffraction pattern of such a crystal, as measured by Matthias F. Groh (RG Prof. Ruck, TU Dresden). (Picture submitted by Dr. Pascal Hartmann.)
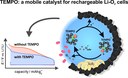
Due to their high energy densities aprotic lithium-oxygen batteries are a promising energy storage system for future applications. However, the major drawback of this battery system are the high charging overpotentials which cause a significant energy loss and a poor cycle life. Coworkers of the Janek group recently showed that the use of dissolved TEMPO (2,2,6,6-Tetramethylpiperidinyloxyl) leads to a distinct reduction of the charging overpotentials and to an increased cycle life, cf. Bergner et al. J. Am. Chem. Soc. 2014, 136, 15054. The picture of the month shows the cycling profile of lithium oxygen cells with and without dissolved TEMPO as well as the proposed charging mechanism. (Picture submitted by Benjamin Bergner.)