Gallery of Pictures from 2016
The picture shows a scheme and a scanning electron microscope (SEM) image of a sample consisting of a sapphire substrate, thermally evaporated gold electrodes, a „LiPON“ solid electrolyte layer and thermally evaporated lithium electrodes. On this sample impedance measurements have been performed. The SEM image shows Top left: Sapphire|Gold|”LiPON”|Li Top right: Sapphire|Gold|”LiPON”Bottom right: Sapphire|”LiPON”Bottom left: Sapphire|”LiPON”|LiThe image impressively shows how the morphology of the electrolyte layer depends on the substrate on which it is deposited. In contact with ambient atmosphere, “LiPON” tends to form flower-like structures which contain lithium carbonate. The evolution of these structures seems to be inhibited if the electrolyte film is deposited on gold.
Lights on! The picture shows an all-solid-state battery that was assembled by compaction of three different components. In contrast to commercially available lithium ion batteries, where an organic liquid electrolyte comes into operation, a sulfur-based solid is used here to separate the electrodes and prevent a short circuit. Concurrently, this inorganic ion conductor ensures sufficient transport of lithium ions between the electrodes – the LED shines.The Janek group conducts research on all-solid-state batteries within the framework of different research projects, like the BASF scientific network “electrochemistry and batteries“, the LOEWE priority program “STORE-E“ (by the Hessen State Ministry of Higher Education, Research and the Arts), as well as the BMBF (Federal Ministry of Education and Research) -granted projects “ProSoLitBat“ and “FELIZIA“. We place our main focus on the elucidation of phenomena that take place at the interfaces between solid electrolyte and the electrodes. (Picture submitted by Dominik Weber and Wenbo Zhang.)
In the week between 29 February and 4 March our group was in Kleinwalsertal for the spring seminar. Besides scientific talks and workshops regarding internal organization there was also room for free time: On Wednesday we went skiing and hiking, on Thursday evening one could take part in a torch-lit hike. (Picture submitted by Carsten Fiedler.)
Peeking inside batteries with X-rays – We show the cathode of a Na/O2 battery in which sodium superoxide is deposited as discharge product. Sodium superoxide is usually observed as cubical particles with up to 20 µm particle size, but can not be monitored during operation directly inside the battery because it is covered by the battery housing and other materials. We visualize these particles (color gradient as measure for particle volume) by means of synchrotron X-ray transmission tomography of a fully discharge battery cathode. Measurements were conducted in cooperation with the Helmholtz-Zentrum Berlin für Materialien und Energie at the electron storage ring BESSY II, and were recently published (http://www.nature.com/articles/srep24288).Electrically rechargeable metal/oxygen batteries, such as the Na/O2 battery, have been considered as possible future energy storage devices. Metal/oxygen batteries are investigated intensively in the Janek research group within the framework of several third-party funded research projects, such as the BASF-Network “Electrochemistry and Batteries”, and the BMBF-Project “BenchBatt”. Main focus is thereby on gaining understanding of the electrochemical and chemical processes and thus on improving cell performance. (Picture submitted by Conrad Bender and Daniel Schröder.)
Insight into the crystallization of amorphous glassesInformation on interatomic distances in glassy inorganic solids can be revealed by X-ray pair-distribution-function analysis (X-PDF). The lithium superionic conductor Li7P3S11 can be crystallized from the amorphous (Li2S)70(P2S5)30 glass. The ionic conductivity of the glass-ceramic (σ25 ≈ 10-2 Scm-1) is able to compete with pertinent liquid battery electrolytes. In the PDF, only sharp peaks corresponding to the first coordination sphere of thio-phosphate tetrahedra at 2.03 Å, (P-S-bonding length, green) and 3.37 Å (S-S-bonding length, blue) are apparent because there is no long-range ordering of the atoms. In contrast, intensive signals can be found far beyond 20 Å for the metastable glass-ceramic Li7P3S11 as well as the thermodynamic stable degradation product Li4P2S6.(Picture submitted by Christian Dietrich, Dominik Weber and Wolfgang Zeier; data collection was performed at the 11-ID-B beamline of the Argonne National Laboratory.)
All-solid-state battery (ASSB) has been drawn enough attention as one of the most potential battery systems for the application in electric vehicles in the near future. Recently, a successful running ASSB has been developed in our group. The ASSB contains a composite cathode, a solid electrolyte and a metal anode, as depicted in Fig a. The good compact of the materials has been achieved with a simple processing technique. The ASSB shows a comparable cycle performance to traditional Li-ion battery with the same active material and organic liquid electrolyte. A high capacity retention has been achieved after 100 cycles at room temperature. The results show a promising future for the further investigation and the eventual application of ASSB. (Picture submitted by Wenbo Zhang.)
For the application of batteries in electric vehicles, it is necessary to raise their efficiency for an increased range. One potential candidate as high-voltage cathode in all-solid-state batteries (ASSBs) is LiCoPO4. The crystal structure of the purple material (left pictures) is built up of chains of edge-sharing cobalt-centered octahedra connected to one another by phosphate tetrahedra. Thin films of the material were prepared in our group by pulsed laser deposition (PLD). The image of the surface taken by confocal microscopy (CLSM; right picture) shows roughness as well as waviness of the film. (Picture submitted by Patrick Hofmann.)
The ionic conductivity of the solid electrolyte Li1+xAlxGe2−x(PO4)3 (LAGP) can be significantly increased by replacing Ge4+ with Al3+ ions, which leads to an increased concentration of Li+ ions. The picture shows a pellet of LAGP that had been prepared by a solid-state reaction. The surface of the pellet was investigated using scanning electron microscopy (SEM) and mostly shows highly crystalline particles with a size of around 100 nm. In addition, some irregularly shaped particles are observed on the surface, which might be the result of a minor fraction of side phases. LAGP crystallizes in the NASICON structure (sodium superionic conductor, Na1+xZr2SixP3−xO12) with space group R-3c (top). Chains of face-sharing LiO6 - and GeO6/AlO6 - octahedra are linked via corner-sharing PO4-tetrahedra resulting in a three-dimensional network. (Picture submitted by Manuel Weiß.)
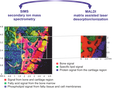
Is Osteoporosis the Obesity of Bone? Correlation between two mass spectrometry imaging methods is supposed to reveal insights into the role of lipids in the disease state of osteoporosis. In cooperation with Prof Wenisch‘s group from veterinary antatomy and Prof Spengler‘s group from analytical chemistry, the identity and distribution of lipids in native bone sections of osteoporotic and control donors is determined and compared by ToF-SIMS & MALDI. The picture shows an exemplary measurement of a control donor. (Picture submitted by Kaija Schaepe.)
In view of the fact that the establishment of alternative energy sources is coupled to the storage of the so generated energy different concepts of stationary energy storage are being investigated at the moment. Besides conventional battery systems like lithium-ion batteries alternative storage solutions that are based on abundant and cost efficient materials enter the limelight. For example several organic molecules that can be obtained by many kinds of resources can be optimized for their application in electrochemical cells. The desired molecules for this application are being defined within the scope of a collaboration between the Physical-Chemical- and the Organic Institute of the Justus-Liebig University by the investigation of the correlation between their structure and electrochemical properties. One important way to have an influence on these properties is to vary the pH value of the electrolyte the electrochemically active species is dissolved in. By modifying it the potential of the electron transfer can be varied to optimize the compound for the application in different environments. The image shows the influence of the pH value on the compounds charge transfer characteristic by shifting its potential. This is illustrated by cyclic voltammogramms that have been measured versus a Ag/AgCl reference electrode within a three-electrode assembly. (Picture submitted by Jonas Hofmann.)